Third row transition metals for the treatment of cancer
- PMID: 25666060
- PMCID: PMC4342973
- DOI: 10.1098/rsta.2014.0185
Third row transition metals for the treatment of cancer
Abstract
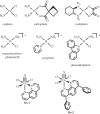
Platinum compounds are a mainstay of cancer chemotherapy, with over 50% of patients receiving platinum. But there is a great need for improvement. Major features of the cisplatin mechanism of action involve cancer cell entry, formation mainly of intrastrand cross-links that bend and unwind nuclear DNA, transcription inhibition and induction of cell-death programmes while evading repair. Recently, we discovered that platinum cross-link formation is not essential for activity. Monofunctional Pt compounds such as phenanthriplatin, which make only a single bond to DNA nucleobases, can be far more active and effective against a range of tumour types. Without a cross-link-induced bend, monofunctional complexes can be accommodated in the major groove of DNA. Their biological mechanism of action is similar to that of cisplatin. These discoveries opened the door to a large family of heavy metal-based drug candidates, including those of Os and Re, as will be described.
Keywords: anti-cancer; monofunctional; osmium; platinum; transition metal.
© 2015 The Author(s) Published by the Royal Society. All rights reserved.
Figures






References
-
- Howlader N, et al. 2012. SEER cancer statistics review, 1975–2009. Bethesda, MD: National Cancer Institute.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

