Cilostazol attenuates the severity of peripheral arterial occlusive disease in patients with type 2 diabetes: the role of plasma soluble receptor for advanced glycation end-products
- PMID: 25666934
- PMCID: PMC4512567
- DOI: 10.1007/s12020-015-0545-6
Cilostazol attenuates the severity of peripheral arterial occlusive disease in patients with type 2 diabetes: the role of plasma soluble receptor for advanced glycation end-products
Abstract
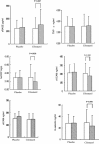
Recent studies have demonstrated that the plasma soluble receptor for advanced glycation end-products (sRAGE) play a major role in developing macrovascular complications of type 2 diabetes, including peripheral arterial occlusion disease (PAOD). Cilostazol is an antiplatelet, antithrombotic agent, which has been used for the treatment of PAOD. We hypothesized that cilostazol attenuates the severity of PAOD in patients with type 2 diabetes through the augmentation of plasma sRAGE. Ninety type 2 diabetic patients with PAOD defined as intermittent claudication with ankle-brachial index (ABI) ≦0.9 were recruited for an open-labeled, placebo-controlled study for 52 weeks with oral cilostazol 100 mg twice daily (n = 45) or placebo (n = 45). Fasting plasma sRAGE, endothelial variables of E-selectin, soluble vascular cell adhesion molecule-1 (sVCAM-1), and inflammatory markers of high-sensitivity C-reactive protein (hsCRP) and tumor necrosis factor-α (TNF-α) were determined. After completely the 52-week treatment program, the ABI values were elevated in cilostazol group (P < 0.001). The plasma sRAGE was significantly increased (P = 0.007), and hsCRP, sVCAM, and E-selectin concentrations were significantly decreased (P = 0.028, <0.001 and <0.001, respectively) with cilostazol treatment. In a partial correlation analysis with adjustments for sex and age, the net change of sRAGE significantly correlated with the change of ABI in the cilostazol group (P = 0.043). In a stepwise multiple regression model, only the change with regards to sRAGE was significantly associated with the change of ABI (P = 0.046). Our results suggest that cilostazol may effectively attenuate the severity of PAOD in patients with type 2 diabetes. Plasma sRAGE plays a role as an independent predictor for improving the index of PAOD.
Figures

References
-
- Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

