Auxin activity: Past, present, and future
- PMID: 25667071
- PMCID: PMC4854432
- DOI: 10.3732/ajb.1400285
Auxin activity: Past, present, and future
Abstract
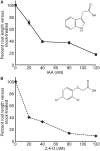
Long before its chemical identity was known, the phytohormone auxin was postulated to regulate plant growth. In the late 1800s, Sachs hypothesized that plant growth regulators, present in small amounts, move differentially throughout the plant to regulate growth. Concurrently, Charles Darwin and Francis Darwin were discovering that light and gravity were perceived by the tips of shoots and roots and that the stimulus was transmitted to other tissues, which underwent a growth response. These ideas were improved upon by Boysen-Jensen and Paál and were later developed into the Cholodny-Went hypothesis that tropisms were caused by the asymmetric distribution of a growth-promoting substance. These observations led to many efforts to identify this elusive growth-promoting substance, which we now know as auxin. In this review of auxin field advances over the past century, we start with a seminal paper by Kenneth Thimann and Charles Schneider titled "The relative activities of different auxins" from the American Journal of Botany, in which they compare the growth altering properties of several auxinic compounds. From this point, we explore the modern molecular understanding of auxin-including its biosynthesis, transport, and perception. Finally, we end this review with a discussion of outstanding questions and future directions in the auxin field. Over the past 100 yr, much of our progress in understanding auxin biology has relied on the steady and collective advance of the field of auxin researchers; we expect that the next 100 yr of auxin research will likewise make many exciting advances.
Keywords: auxin; auxin history; metabolism; signaling; transport.
© 2015 Botanical Society of America, Inc.
Figures



References
-
- Barbez E, Kubes M, Rolcik J, Beziat C, Pencik A, Wang B, Rosquete MR, et al. A novel putative auxin carrier family regulates intracellular auxin homeostasis in plants. Nature. 2012;485:119–122. - PubMed
-
- Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, et al. Arabidopsis AUX1 gene: A permease-like regulator of root gravitropism. Science. 1996;273:948–950. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

