Molecular profiling and targeted therapy for advanced thoracic malignancies: a biomarker-derived, multiarm, multihistology phase II basket trial
- PMID: 25667274
- PMCID: PMC4356709
- DOI: 10.1200/JCO.2014.58.2007
Molecular profiling and targeted therapy for advanced thoracic malignancies: a biomarker-derived, multiarm, multihistology phase II basket trial
Abstract
Purpose: We conducted a basket clinical trial to assess the feasibility of such a design strategy and to independently evaluate the effects of multiple targeted agents against specific molecular aberrations in multiple histologic subtypes concurrently.
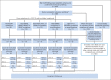
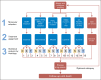
Patients and methods: We enrolled patients with advanced non-small-cell lung cancer (NSCLC), small-cell lung cancer, and thymic malignancies who underwent genomic characterization of oncogenic drivers. Patients were enrolled onto a not-otherwise-specified arm and treated with standard-of-care therapies or one of the following five biomarker-matched treatment groups: erlotinib for EGFR mutations; selumetinib for KRAS, NRAS, HRAS, or BRAF mutations; MK2206 for PIK3CA, AKT, or PTEN mutations; lapatinib for ERBB2 mutations or amplifications; and sunitinib for KIT or PDGFRA mutations or amplification.
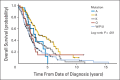
Results: Six hundred forty-seven patients were enrolled, and 88% had their tumors tested for at least one gene. EGFR mutation frequency was 22.1% in NSCLC, and erlotinib achieved a response rate of 60% (95% CI, 32.3% to 83.7%). KRAS mutation frequency was 24.9% in NSCLC, and selumetinib failed to achieve its primary end point, with a response rate of 11% (95% CI, 0% to 48%). Completion of accrual to all other arms was not feasible. In NSCLC, patients with EGFR mutations had the longest median survival (3.51 years; 95% CI, 2.89 to 5.5 years), followed by those with ALK rearrangements (2.94 years; 95% CI, 1.66 to 4.61 years), those with KRAS mutations (2.3 years; 95% CI, 2.3 to 2.17 years), those with other genetic abnormalities (2.17 years; 95% CI, 1.3 to 2.74 years), and those without an actionable mutation (1.85 years; 95% CI, 1.61 to 2.13 years).
Conclusion: This basket trial design was not feasible for many of the arms with rare mutations, but it allowed the study of the genetics of less common malignancies.
Trial registration: ClinicalTrials.gov NCT01306045.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures

Comment in
-
Basket trials and the evolution of clinical trial design in an era of genomic medicine.J Clin Oncol. 2015 Mar 20;33(9):975-7. doi: 10.1200/JCO.2014.59.8433. Epub 2015 Feb 9. J Clin Oncol. 2015. PMID: 25667288 No abstract available.
-
Basket Trials: Just the End of the First Quarter.J Clin Oncol. 2015 Sep 1;33(25):2823-4. doi: 10.1200/JCO.2015.62.1516. Epub 2015 Jul 13. J Clin Oncol. 2015. PMID: 26169614 No abstract available.
-
Reply to A. Stenzinger et al.J Clin Oncol. 2015 Sep 1;33(25):2824. doi: 10.1200/JCO.2015.62.7323. Epub 2015 Jul 13. J Clin Oncol. 2015. PMID: 26169619 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

