BRAF mutation and CDKN2A deletion define a clinically distinct subgroup of childhood secondary high-grade glioma
- PMID: 25667294
- PMCID: PMC4356711
- DOI: 10.1200/JCO.2014.58.3922
BRAF mutation and CDKN2A deletion define a clinically distinct subgroup of childhood secondary high-grade glioma
Abstract
Purpose: To uncover the genetic events leading to transformation of pediatric low-grade glioma (PLGG) to secondary high-grade glioma (sHGG).
Patients and methods: We retrospectively identified patients with sHGG from a population-based cohort of 886 patients with PLGG with long clinical follow-up. Exome sequencing and array CGH were performed on available samples followed by detailed genetic analysis of the entire sHGG cohort. Clinical and outcome data of genetically distinct subgroups were obtained.
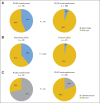
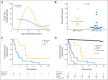
Results: sHGG was observed in 2.9% of PLGGs (26 of 886 patients). Patients with sHGG had a high frequency of nonsilent somatic mutations compared with patients with primary pediatric high-grade glioma (HGG; median, 25 mutations per exome; P = .0042). Alterations in chromatin-modifying genes and telomere-maintenance pathways were commonly observed, whereas no sHGG harbored the BRAF-KIAA1549 fusion. The most recurrent alterations were BRAF V600E and CDKN2A deletion in 39% and 57% of sHGGs, respectively. Importantly, all BRAF V600E and 80% of CDKN2A alterations could be traced back to their PLGG counterparts. BRAF V600E distinguished sHGG from primary HGG (P = .0023), whereas BRAF and CDKN2A alterations were less commonly observed in PLGG that did not transform (P < .001 and P < .001 respectively). PLGGs with BRAF mutations had longer latency to transformation than wild-type PLGG (median, 6.65 years [range, 3.5 to 20.3 years] v 1.59 years [range, 0.32 to 15.9 years], respectively; P = .0389). Furthermore, 5-year overall survival was 75% ± 15% and 29% ± 12% for children with BRAF mutant and wild-type tumors, respectively (P = .024).
Conclusion: BRAF V600E mutations and CDKN2A deletions constitute a clinically distinct subtype of sHGG. The prolonged course to transformation for BRAF V600E PLGGs provides an opportunity for surgical interventions, surveillance, and targeted therapies to mitigate the outcome of sHGG.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures

Comment in
-
Malignant transformation of low-grade gliomas in children: lessons learned from rare medical events.J Clin Oncol. 2015 Mar 20;33(9):978-9. doi: 10.1200/JCO.2014.60.1823. Epub 2015 Feb 9. J Clin Oncol. 2015. PMID: 25667289 No abstract available.
References
-
- Broniscer A, Baker SJ, West AN, et al. Clinical and molecular characteristics of malignant transformation of low-grade glioma in children. J Clin Oncol. 2007;25:682–689. - PubMed
-
- Krieger MD, Gonzalez-Gomez I, Levy ML, et al. Recurrence patterns and anaplastic change in a long-term study of pilocytic astrocytomas. Pediatr Neurosurg. 1997;27:1–11. - PubMed
-
- Dirks PB, Jay V, Becker LE, et al. Development of anaplastic changes in low-grade astrocytomas of childhood. Neurosurgery. 1994;34:68–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

