Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma
- PMID: 25667295
- PMCID: PMC5089162
- DOI: 10.1200/JCO.2014.56.2736
Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma
Abstract
Purpose: To provide a more precise estimate of long-term survival observed for ipilimumab-treated patients with advanced melanoma, we performed a pooled analysis of overall survival (OS) data from multiple studies.
Methods: The primary analysis pooled OS data for 1,861 patients from 10 prospective and two retrospective studies of ipilimumab, including two phase III trials. Patients were previously treated (n = 1,257) or treatment naive (n = 604), and the majority of patients received ipilimumab 3 mg/kg (n = 965) or 10 mg/kg (n = 706). We also conducted a secondary analysis of OS data (n = 4,846) with an additional 2,985 patients from an expanded access program. OS rates were estimated using the Kaplan-Meier method.
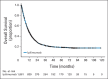
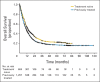
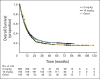
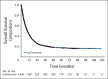
Results: Among 1,861 patients, median OS was 11.4 months (95% CI, 10.7 to 12.1 months), which included 254 patients with at least 3 years of survival follow-up. The survival curve began to plateau around year 3, with follow-up of up to 10 years. Three-year survival rates were 22%, 26%, and 20% for all patients, treatment-naive patients, and previously treated patients, respectively. Including data from the expanded access program, median OS was 9.5 months (95% CI, 9.0 to 10.0 months), with a plateau at 21% in the survival curve beginning around year 3.
Conclusion: To our knowledge, this is the largest analysis of OS to date for ipilimumab-treated patients with advanced melanoma. We observed a plateau in the survival curve, beginning at approximately 3 years, which was independent of prior therapy or ipilimumab dose. These data add to the evidence supporting the durability of long-term survival in ipilimumab-treated patients with advanced melanoma.
Trial registration: ClinicalTrials.gov NCT00032045 NCT00058279 NCT00077532 NCT00094653 NCT00135408 NCT00261365 NCT00289627 NCT00289640 NCT00324155 NCT00623766.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Gauging the Long-Term Benefits of Ipilimumab in Melanoma.J Clin Oncol. 2015 Jun 10;33(17):1865-6. doi: 10.1200/JCO.2014.59.5041. Epub 2015 Feb 9. J Clin Oncol. 2015. PMID: 25667273 No abstract available.
References
-
- Atkins MB, Kunkel L, Sznol M, et al. High-dose recombinant interleukin-2 therapy with metastatic melanoma: Long-term survival update. Cancer J Sci Am. 2000;6(suppl 1):S11–S14. - PubMed
-
- Melero I, Hervas-Stubbs S, Glennie M, et al. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007;7:95–106. - PubMed
-
- Hoos A, Ibrahim R, Korman A, et al. Development of ipilimumab: Contribution to a new paradigm for cancer immunotherapy. Semin Oncol. 2010;37:533–546. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

