2-cysteine peroxiredoxins and thylakoid ascorbate peroxidase create a water-water cycle that is essential to protect the photosynthetic apparatus under high light stress conditions
- PMID: 25667319
- PMCID: PMC4378167
- DOI: 10.1104/pp.114.255356
2-cysteine peroxiredoxins and thylakoid ascorbate peroxidase create a water-water cycle that is essential to protect the photosynthetic apparatus under high light stress conditions
Abstract
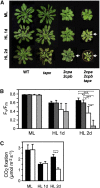
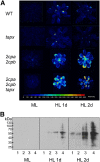
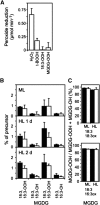
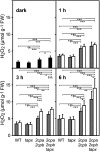
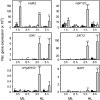
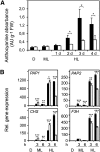
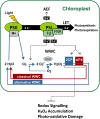
Different peroxidases, including 2-cysteine (2-Cys) peroxiredoxins (PRXs) and thylakoid ascorbate peroxidase (tAPX), have been proposed to be involved in the water-water cycle (WWC) and hydrogen peroxide (H2O2)-mediated signaling in plastids. We generated an Arabidopsis (Arabidopsis thaliana) double-mutant line deficient in the two plastid 2-Cys PRXs (2-Cys PRX A and B, 2cpa 2cpb) and a triple mutant deficient in 2-Cys PRXs and tAPX (2cpa 2cpb tapx). In contrast to wild-type and tapx single-knockout plants, 2cpa 2cpb double-knockout plants showed an impairment of photosynthetic efficiency and became photobleached under high light (HL) growth conditions. In addition, double-mutant plants also generated elevated levels of superoxide anion radicals, H2O2, and carbonylated proteins but lacked anthocyanin accumulation under HL stress conditions. Under HL conditions, 2-Cys PRXs seem to be essential in maintaining the WWC, whereas tAPX is dispensable. By comparison, this HL-sensitive phenotype was more severe in 2cpa 2cpb tapx triple-mutant plants, indicating that tAPX partially compensates for the loss of functional 2-Cys PRXs by mutation or inactivation by overoxidation. In response to HL, H2O2- and photooxidative stress-responsive marker genes were found to be dramatically up-regulated in 2cpa 2cpb tapx but not 2cpa 2cpb mutant plants, suggesting that HL-induced plastid to nucleus retrograde photooxidative stress signaling takes place after loss or inactivation of the WWC enzymes 2-Cys PRX A, 2-Cys PRX B, and tAPX.
© 2015 American Society of Plant Biologists. All Rights Reserved.
Figures

References
-
- Asada K. (1999) THE WATER-WATER CYCLE IN CHLOROPLASTS: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50: 601–639 - PubMed
-
- Bauer H, Ache P, Lautner S, Fromm J, Hartung W, Al-Rasheid KA, Sonnewald S, Sonnewald U, Kneitz S, Lachmann N, et al. (2013) The stomatal response to reduced relative humidity requires guard cell-autonomous ABA synthesis. Curr Biol 23: 53–57 - PubMed
-
- Birtic S, Ksas B, Genty B, Mueller MJ, Triantaphylidès C, Havaux M (2011) Using spontaneous photon emission to image lipid oxidation patterns in plant tissues. Plant J 67: 1103–1115 - PubMed
-
- Bonfig KB, Schreiber U, Gabler A, Roitsch T, Berger S (2006) Infection with virulent and avirulent P. syringae strains differentially affects photosynthesis and sink metabolism in Arabidopsis leaves. Planta 225: 1–12 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

