Rhodopsin kinase and recoverin modulate phosphodiesterase during mouse photoreceptor light adaptation
- PMID: 25667411
- PMCID: PMC4338159
- DOI: 10.1085/jgp.201411273
Rhodopsin kinase and recoverin modulate phosphodiesterase during mouse photoreceptor light adaptation
Abstract
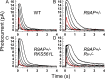
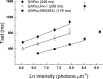
Light stimulates rhodopsin in a retinal rod to activate the G protein transducin, which binds to phosphodiesterase (PDE), relieving PDE inhibition and decreasing guanosine 3',5'-cyclic monophosphate (cGMP) concentration. The decrease in cGMP closes outer segment channels, producing the rod electrical response. Prolonged exposure to light decreases sensitivity and accelerates response kinetics in a process known as light adaptation, mediated at least in part by a decrease in outer segment Ca(2+). Recent evidence indicates that one of the mechanisms of adaptation in mammalian rods is down-regulation of PDE. To investigate the effect of light and a possible role of rhodopsin kinase (G protein-coupled receptor kinase 1 [GRK1]) and the GRK1-regulating protein recoverin on PDE modulation, we used transgenic mice with decreased expression of GTPase-accelerating proteins (GAPs) and, consequently, a less rapid decay of the light response. This slowed decay made the effects of genetic manipulation of GRK1 and recoverin easier to observe and interpret. We monitored the decay of the light response and of light-activated PDE by measuring the exponential response decay time (τREC) and the limiting time constant (τD), the latter of which directly reflects light-activated PDE decay under the conditions of our experiments. We found that, in GAP-underexpressing rods, steady background light decreased both τREC and τD, and the decrease in τD was nearly linear with the decrease in amplitude of the outer segment current. Background light had little effect on τREC or τD if the gene for recoverin was deleted. Moreover, in GAP-underexpressing rods, increased GRK1 expression or deletion of recoverin produced large and highly significant accelerations of τREC and τD. The simplest explanation of our results is that Ca(2+)-dependent regulation of GRK1 by recoverin modulates the decay of light-activated PDE, and that this modulation is responsible for acceleration of response decay and the increase in temporal resolution of rods in background light.
© 2015 Chen et al.
Figures





Similar articles
-
Role of recoverin in rod photoreceptor light adaptation.J Physiol. 2018 Apr 15;596(8):1513-1526. doi: 10.1113/JP275779. Epub 2018 Mar 5. J Physiol. 2018. PMID: 29435986 Free PMC article.
-
Modulation of mouse rod response decay by rhodopsin kinase and recoverin.J Neurosci. 2012 Nov 7;32(45):15998-6006. doi: 10.1523/JNEUROSCI.1639-12.2012. J Neurosci. 2012. PMID: 23136436 Free PMC article.
-
Background light produces a recoverin-dependent modulation of activated-rhodopsin lifetime in mouse rods.J Neurosci. 2010 Jan 27;30(4):1213-20. doi: 10.1523/JNEUROSCI.4353-09.2010. J Neurosci. 2010. PMID: 20107049 Free PMC article.
-
Adaptation of mammalian photoreceptors to background light: putative role for direct modulation of phosphodiesterase.Mol Neurobiol. 2011 Dec;44(3):374-82. doi: 10.1007/s12035-011-8205-1. Epub 2011 Sep 16. Mol Neurobiol. 2011. PMID: 21922272 Free PMC article. Review.
-
Adaptation in vertebrate photoreceptors.Physiol Rev. 2001 Jan;81(1):117-151. doi: 10.1152/physrev.2001.81.1.117. Physiol Rev. 2001. PMID: 11152756 Review.
Cited by
-
Identification and Characterization of a Rhodopsin Kinase Gene in the Suckers of Octopus vulgaris: Looking around Using Arms?Biology (Basel). 2021 Sep 19;10(9):936. doi: 10.3390/biology10090936. Biology (Basel). 2021. PMID: 34571813 Free PMC article.
-
Role of recoverin in rod photoreceptor light adaptation.J Physiol. 2018 Apr 15;596(8):1513-1526. doi: 10.1113/JP275779. Epub 2018 Mar 5. J Physiol. 2018. PMID: 29435986 Free PMC article.
-
Phosphorylation at Serine 21 in G protein-coupled receptor kinase 1 (GRK1) is required for normal kinetics of dark adaption in rod but not cone photoreceptors.FASEB J. 2020 Feb;34(2):2677-2690. doi: 10.1096/fj.201902535R. Epub 2019 Dec 30. FASEB J. 2020. PMID: 31908030 Free PMC article.
-
The Molecular Mechanism of Retina Light Injury Focusing on Damage from Short Wavelength Light.Oxid Med Cell Longev. 2022 Apr 19;2022:8482149. doi: 10.1155/2022/8482149. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35498134 Free PMC article. Review.
-
Light responses of mammalian cones.Pflugers Arch. 2021 Sep;473(9):1555-1568. doi: 10.1007/s00424-021-02551-0. Epub 2021 Mar 19. Pflugers Arch. 2021. PMID: 33742309 Free PMC article. Review.
References
-
- Balasubramanian N., Levay K., Keren-Raifman T., Faurobert E., and Slepak V.Z.. 2001. Phosphorylation of the regulator of G protein signaling RGS9-1 by protein kinase A is a potential mechanism of light- and Ca2+-mediated regulation of G protein function in photoreceptors. Biochemistry. 40:12619–12627 10.1021/bi015624b - DOI - PubMed
-
- Brindley G.S.1970. Physiology of the retina and visual pathway. Williams and Wilkins, Baltimore, MD: 315 pp.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

