Effect of prior cancer on outcomes in advanced lung cancer: implications for clinical trial eligibility and accrual
- PMID: 25667420
- PMCID: PMC4402360
- DOI: 10.1093/jnci/djv002
Effect of prior cancer on outcomes in advanced lung cancer: implications for clinical trial eligibility and accrual
Abstract
Background: Prior cancer is a common exclusion criterion in lung cancer trials. This practice reflects concerns that prior cancer may affect trial conduct or outcomes. However, the impact of prior cancer on survival in lung cancer is not known.
Methods: We identified patients older than age 65 years with stage IV lung cancer diagnosed between 1992 and 2009 in the Surveillance, Epidemiology, and End Results-Medicare linked registry. Prior cancer was characterized by type, stage, and timing. All-cause and lung cancer-specific survival were compared between patients with and without prior cancer using propensity score-adjusted Cox regression.
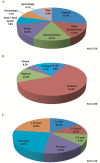
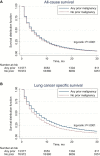
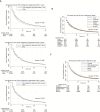
Results: Overall, 102 929 patients with stage IV lung cancer were identified, of whom 14.7% had a history of prior cancer. More than two-thirds (76.0%) of prior cancers were localized or regional stage; most were diagnosed five or fewer years prior to the lung cancer diagnosis. In propensity score-adjusted analysis, patients with prior cancer had better all-cause (hazard ratio [HR] = 0.93, 95% confidence interval [CI] = 0.91 to 0.94) and lung cancer-specific (HR = 0.81, 95% CI = 0.79 to 0.82) survival. In a simulated clinical trial-eligible population (age <75 years, no comorbidity, treated with chemotherapy), similar trends were noted. In subset analyses according to stage, type, and timing of prior cancer, no group of patients with prior cancer had inferior survival compared with patients without prior cancer.
Conclusion: Among patients with stage IV lung cancer, prior cancer does not convey an adverse effect on clinical outcomes, regardless of prior cancer stage, type, or timing. Broader inclusion in clinical trials of advanced lung cancer patients with a history of prior cancer should be considered.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Howerton MW, Gibbons MC, Baffi CR, et al. Provider roles in the recruitment of underrepresented populations to cancer clinical trials. Cancer. 2007;109(3):465–476. - PubMed
-
- Friedman MA, Cain DF. National Cancer Institute sponsored cooperative clinical trials. Cancer. 1990;65(10 Suppl):2376–2382. - PubMed
-
- Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726. - PubMed
-
- Lara PN, Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001;19(6):1728–1733. - PubMed
-
- Lemieux J, Goodwin PJ, Pritchard KI, et al. Identification of cancer care and protocol characteristics associated with recruitment in breast cancer clinical trials. J Clin Oncol. 2008;26(27):4458–4465. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

