Naringenin suppresses K562 human leukemia cell proliferation and ameliorates Adriamycin-induced oxidative damage in polymorphonuclear leukocytes
- PMID: 25667616
- PMCID: PMC4316947
- DOI: 10.3892/etm.2015.2185
Naringenin suppresses K562 human leukemia cell proliferation and ameliorates Adriamycin-induced oxidative damage in polymorphonuclear leukocytes
Abstract
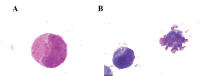
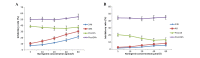
Treatments for leukemia remain unsatisfactory. Conventional chemotherapy agents that aim to kill tumor cells may also damage normal cells and thus result in severe side-effects. Naringenin, a natural polyphenolic compound with antioxidant effects, has been revealed to have significant antitumor effects with low toxicity in preliminary studies. Thus, it is considered as one of the most promising flavonoids in the treatment of leukemia. In the present study, the effects of naringenin on the K562 human leukemia cell line and the underlying mechanisms were explored in vitro. In addition, human peripheral blood polymorphonuclear leukocytes (PMNs) were used as a normal control in order to evaluate the effects of naringenin on normal granulocytes and in the mediation of Adriamycin (ADM)-induced oxidative damage. The results revealed that K562 proliferation was significantly inhibited by naringenin in a time- and concentration-dependent manner; however, minimal cytotoxic effects were observed in PMNs when naringenin was used at concentrations <400 μmol/l. Morphological changes indicative of apoptosis were observed in naringenin-treated K562 cells. Flow cytometric analysis indicated that the K562 cells were arrested in the G0/G1 phase of the cell cycle with a significantly upregulated rate of apoptosis. Furthermore, in the naringenin-treated K562 cells, the labeling index of proliferating cell nuclear antigen was observed to be increased by immunochemical staining, the mRNA and protein expression levels of p21/WAF1 were strongly upregulated in reverse transcription-polymerase chain reaction and western blot analyses, whereas p53 gene expression was not significantly changed. In PMNs to which naringenin (50~80 μmol/l) was added 1 h subsequent to ADM, the cell damage induced by ADM was significantly reduced, coincident with reductions in the levels of reactive oxygen species (ROS) and malondialdehyde (MDA) and increases in the activity of superoxide dismutase and glutathione peroxidase. However, the cytotoxic effect of ADM in K562 cells was not significantly altered by naringenin, and the oxidative stress indices in K562 cells remained stable. In conclusion, the present study revealed the promising value of naringenin in leukemia treatment. Naringenin demonstrated a significant inhibitory effect on the growth of K562 cells but not on normal PMNs. Furthermore, naringenin protected PMNs from ADM-induced oxidative damage at low concentrations. Cell cycle arrest and apoptosis-inducing effects, achieved through p53-independent p21/WAF1 upregulation, are likely to be the mechanism of the antileukemic effects of naringenin, and the protective effect against ADM chemotherapy-induced damage in PMNs may be due to the antioxidant capability of this agent at low concentrations.
Keywords: K562; apoptosis; cell cycle; cell line; gene; naringenin; p21; p53.
Figures






References
-
- Yang C, Zhang X. Incidence survey of leukemia in China. Chin Med Sci J. 1991;6:65–70. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
