NADPH oxidase-dependent production of reactive oxygen species induces endoplasmatic reticulum stress in neutrophil-like HL60 cells
- PMID: 25668518
- PMCID: PMC4323339
- DOI: 10.1371/journal.pone.0116410
NADPH oxidase-dependent production of reactive oxygen species induces endoplasmatic reticulum stress in neutrophil-like HL60 cells
Abstract
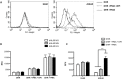
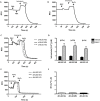
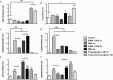
Reactive oxygen species (ROS) primarily produced via NADPH oxidase play an important role for killing microorganisms in neutrophils. In this study we examined if ROS production in Human promyelocytic leukemia cells (HL60) differentiated into neutrophil-like cells (dHL60) induces ER stress and activates the unfolded protein response (UPR). To cause ROS production cells were treated with PMA or by chronic hyperglycemia. Chronic hyperglycemia failed to induce ROS production and did not cause activation of the UPR in dHL60 cells. PMA, a pharmacologic NADPH oxidase activator, induced ER stress in dHL60 cells as monitored by IRE-1 and PERK pathway activation, and this was independent of calcium signaling. The NADPH oxidase inhibitor, DPI, abolished both ROS production and UPR activation. These results show that ROS produced by NADPH oxidase induces ER stress and suggests a close association between the redox state of the cell and the activation of the UPR in neutrophil-like HL60 cells.
Conflict of interest statement
Figures



References
-
- Cassatella MA (1999) Neutrophil-derived proteins: selling cytokines by the pound. Adv Immunol 1999; 73: 369–509. - PubMed
-
- Rossi F, Zatti M (1964) Biochemical aspects of phagocytosis in polymorphonuclear leucocytes. NADH and NADPH oxidation by the granules of resting and phagocytizing cells. Experientia 20(1): 21–23. - PubMed
-
- Savill J, Haslett C (1995) Granulocyte clearance by apoptosis in the resolution of inflammation. Semin Cell Biol 6(6): 385–393. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

