Immunogenicity and safety of currently available Japanese encephalitis vaccines: a systematic review
- PMID: 25668666
- PMCID: PMC4514081
- DOI: 10.4161/21645515.2014.980197
Immunogenicity and safety of currently available Japanese encephalitis vaccines: a systematic review
Abstract
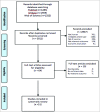
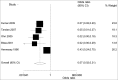
A number of Japanese encephalitis (JE) vaccines have been used for preventing Japanese encephalitis around the world. We here reviewed the immunogenicity and safety of the currently available Japanese encephalitis vaccines. We searched Pubmed, Embase, Web of Science, the Cochrane Library and other online databases up to March 25, 2014 for studies focusing on currently used JE vaccines in any language. The primary outcomes were the seroconversion rate against JEV and adverse events. Meta-analysis was performed for the primary outcome when available. A total of 51 articles were included. Studies were grouped on the basic types of vaccines. This systematic review led to 2 aspects of the conclusions. On one hand, all the currently available JE vaccines are safe and effective. On the other hand, the overall of JE vaccine evaluation is disorganized, the large variation in study designs, vaccine types, schedules, doses, population and few hand-to-hand trails, make direct comparisons difficult. In order to make a more evidence-based decision on optimizing the JE vaccine, it is warranted to standardize the JE vaccine evaluation research.
Keywords: ACIP, The Advisory Committee on Immunization Practices; AEs, Adverse events; CENTRAL, Cochrane Central Register of Controlled Trials; CIs, Confidence intervals; DARE, Database of Abstracts of Reviews of Effects; GMTs, Geometric mean titers; HAART, Highly active antiretroviral therapy; JE, Japanese encephalitis; JE-CV, Chimeric live-attenuated JE vaccine; JEV, Japanese encephalitis virus; Japanese encephalitis vaccine; LILACS, Latin American and Caribbean Health Sciences Literature; MBJEV, Mouse brain–derived inactivated vaccines; MMR, Measles mumps rubella vaccine; NIP, National Immunization Program; NOS, Newcastle–Ottawa scale; ORs, Odd ratios; PHK, Primary hamster kidney cells; PRNT50, Plaque-reduction neutralization tests; RCTs, Randomized controlled trials; TBE, Tick-borne encephalitis vaccine; TGPO, Thai Governmental Pharmaceutical Organization; WHO, World Health Organization; YF-VAX, Yellow fever vaccine; YFV, Yellow fever virus; YFV17D, Yellow fever virus 17D vaccine strain; immunogenicity; meta-analysis; safety; systematic review.
Figures
References
-
- Solomon T. Control of Japanese encephalitis–within our grasp? N Engl J Med 2006; 355:869-71; PMID: 16943399; http://dx.doi.org/10.1056/NEJMp058263 - DOI - PubMed
-
- Campbell GL, Hills SL, Fischer M, Jacobson JA, Hoke CH, Hombach JM, Marfin AA, Solomon T, Tsai TF, Tsu VD, et al. . Estimated global incidence of Japanese encephalitis: a systematic review. Bull World Health Organ 2011; 89:766-74, 74A-74E; PMID:22084515; http://dx.doi.org/10.2471/BLT.10.085233 - DOI - PMC - PubMed
-
- WHO position paper Japanese encephalitis vaccines. Releve epidemiologique hebdomadaire / Section d'hygiene du Secretariat de la Societe des Nations=Weekly epidemiological record / Health Section of the Secretariat of the League of Nations 2006; 81:331-40
-
- Appaiahgari MB, Vrati S. IMOJEV((R)): a Yellow fever virus-based novel Japanese encephalitis vaccine. Expert Rev Vaccines 2010; 9:1371-84; PMID:21105774; http://dx.doi.org/10.1586/erv.10.139 - DOI - PubMed
-
- Centers for Disease Control and PreventionUpdate on Japanese encephalitis vaccine for children: United States, May 2011. MMWR Morbidity and mortality weekly report 2011; 60:664-5
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical