Motoneuron axon pathfinding errors in zebrafish: differential effects related to concentration and timing of nicotine exposure
- PMID: 25668718
- PMCID: PMC4567840
- DOI: 10.1016/j.taap.2015.01.022
Motoneuron axon pathfinding errors in zebrafish: differential effects related to concentration and timing of nicotine exposure
Abstract
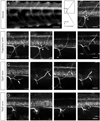
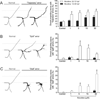
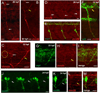
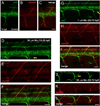
Nicotine exposure during embryonic stages of development can affect many neurodevelopmental processes. In the developing zebrafish, exposure to nicotine was reported to cause axonal pathfinding errors in the later born secondary motoneurons (SMNs). These alterations in SMN axon morphology coincided with muscle degeneration at high nicotine concentrations (15-30 μM). Previous work showed that the paralytic mutant zebrafish known as sofa potato exhibited nicotine-induced effects onto SMN axons at these high concentrations but in the absence of any muscle deficits, indicating that pathfinding errors could occur independent of muscle effects. In this study, we used varying concentrations of nicotine at different developmental windows of exposure to specifically isolate its effects onto subpopulations of motoneuron axons. We found that nicotine exposure can affect SMN axon morphology in a dose-dependent manner. At low concentrations of nicotine, SMN axons exhibited pathfinding errors, in the absence of any nicotine-induced muscle abnormalities. Moreover, the nicotine exposure paradigms used affected the 3 subpopulations of SMN axons differently, but the dorsal projecting SMN axons were primarily affected. We then identified morphologically distinct pathfinding errors that best described the nicotine-induced effects on dorsal projecting SMN axons. To test whether SMN pathfinding was potentially influenced by alterations in the early born primary motoneuron (PMN), we performed dual labeling studies, where both PMN and SMN axons were simultaneously labeled with antibodies. We show that only a subset of the SMN axon pathfinding errors coincided with abnormal PMN axonal targeting in nicotine-exposed zebrafish. We conclude that nicotine exposure can exert differential effects depending on the levels of nicotine and developmental exposure window.
Keywords: Axonal pathfinding; Dual labeling; Embryonic spinal cord; Muscle degeneration.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures






Similar articles
-
Uncoupling nicotine mediated motoneuron axonal pathfinding errors and muscle degeneration in zebrafish.Toxicol Appl Pharmacol. 2009 May 15;237(1):29-40. doi: 10.1016/j.taap.2008.06.025. Epub 2008 Jul 23. Toxicol Appl Pharmacol. 2009. PMID: 18694773 Free PMC article.
-
Secondary motoneurons in juvenile and adult zebrafish: axonal pathfinding errors caused by embryonic nicotine exposure.J Comp Neurol. 2009 Jan 20;512(3):305-22. doi: 10.1002/cne.21903. J Comp Neurol. 2009. PMID: 19006183 Free PMC article.
-
The SMN binding protein Gemin2 is not involved in motor axon outgrowth.Dev Neurobiol. 2008 Feb 1;68(2):182-94. doi: 10.1002/dneu.20582. Dev Neurobiol. 2008. PMID: 18000835
-
Developmental regulation of subtype-specific motor neuron excitability.Ann N Y Acad Sci. 2010 Jun;1198:201-7. doi: 10.1111/j.1749-6632.2009.05426.x. Ann N Y Acad Sci. 2010. PMID: 20536935 Review.
-
Spontaneous rhythmic activity in early chick spinal cord influences distinct motor axon pathfinding decisions.Brain Res Rev. 2008 Jan;57(1):77-85. doi: 10.1016/j.brainresrev.2007.06.021. Epub 2007 Aug 1. Brain Res Rev. 2008. PMID: 17920131 Free PMC article. Review.
Cited by
-
Functional limb muscle innervation prior to cholinergic transmitter specification during early metamorphosis in Xenopus.Elife. 2018 May 30;7:e30693. doi: 10.7554/eLife.30693. Elife. 2018. PMID: 29845935 Free PMC article.
-
Using Zebrafish to Implement a Course-Based Undergraduate Research Experience to Study Teratogenesis in Two Biology Laboratory Courses.Zebrafish. 2016 Aug;13(4):293-304. doi: 10.1089/zeb.2015.1107. Epub 2016 Feb 1. Zebrafish. 2016. PMID: 26829498 Free PMC article.
References
-
- Beattie CE, Melancon E, Eisen JS. Mutations in the stumpy gene reveal intermediate targets for zebrafish motor axons. Development. 2000;127(12):2653–2662. - PubMed
-
- Behra M, Cousin X, Bertrand C, Vonesch JL, Biellmann D, Chatonnet A, et al. Acetylcholinesterase is required for neuronal and muscular development in the zebrafish embryo. Nature Neuroscience. 2002;5(2):111–118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

