The invertebrate Caenorhabditis elegans biosynthesizes ascorbate
- PMID: 25668719
- PMCID: PMC4357563
- DOI: 10.1016/j.abb.2015.02.002
The invertebrate Caenorhabditis elegans biosynthesizes ascorbate
Abstract
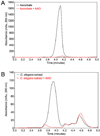
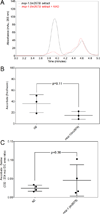
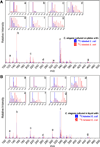
l-Ascorbate, commonly known as vitamin C, serves as an antioxidant and cofactor essential for many biological processes. Distinct ascorbate biosynthetic pathways have been established for animals and plants, but little is known about the presence or synthesis of this molecule in invertebrate species. We have investigated ascorbate metabolism in the nematode Caenorhabditis elegans, where this molecule would be expected to play roles in oxidative stress resistance and as cofactor in collagen and neurotransmitter synthesis. Using high-performance liquid chromatography and gas-chromatography mass spectrometry, we determined that ascorbate is present at low amounts in the egg stage, L1 larvae, and mixed animal populations, with the egg stage containing the highest concentrations. Incubating C. elegans with precursor molecules necessary for ascorbate synthesis in plants and animals did not significantly alter ascorbate levels. Furthermore, bioinformatic analyses did not support the presence in C. elegans of either the plant or the animal biosynthetic pathway. However, we observed the complete (13)C-labeling of ascorbate when C. elegans was grown with (13)C-labeled Escherichia coli as a food source. These results support the hypothesis that ascorbate biosynthesis in invertebrates may proceed by a novel pathway and lay the foundation for a broader understanding of its biological role.
Keywords: Ascorbic acid; Biosynthetic pathway; Caenorhabditis elegans; Invertebrates; Vitamin C.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures






Similar articles
-
L-Ascorbate Biosynthesis Involves Carbon Skeleton Rearrangement in the Nematode Caenorhabditis elegans.Metabolites. 2020 Aug 17;10(8):334. doi: 10.3390/metabo10080334. Metabolites. 2020. PMID: 32824560 Free PMC article.
-
The biosynthesis of ascorbate protects isolated rat hepatocytes from cumene hydroperoxide-mediated oxidative stress.Free Radic Biol Med. 2005 Apr 1;38(7):867-73. doi: 10.1016/j.freeradbiomed.2004.12.006. Free Radic Biol Med. 2005. PMID: 15749382
-
Ethanol-induced differential gene expression and acetyl-CoA metabolism in a longevity model of the nematode Caenorhabditis elegans.Exp Gerontol. 2015 Jan;61:20-30. doi: 10.1016/j.exger.2014.11.010. Epub 2014 Nov 18. Exp Gerontol. 2015. PMID: 25449858 Free PMC article.
-
Prospects for enhancement of the soluble antioxidants, ascorbate and glutathione.Biofactors. 2001;15(2-4):75-8. doi: 10.1002/biof.5520150204. Biofactors. 2001. PMID: 12016329 Review.
-
The role of stress in ageing: research on the nematode, Caenorhabditis elegans.Br J Dermatol. 2005 Dec;153 Suppl 2:1-5. doi: 10.1111/j.1365-2133.2005.06963.x. Br J Dermatol. 2005. PMID: 16280015 Review.
Cited by
-
UPRER-immunity axis acts as physiological food evaluation system that promotes aversion behavior in sensing low-quality food.Elife. 2024 Sep 5;13:RP94181. doi: 10.7554/eLife.94181. Elife. 2024. PMID: 39235964 Free PMC article.
-
Vitamin B12 deficiency results in severe oxidative stress, leading to memory retention impairment in Caenorhabditis elegans.Redox Biol. 2017 Apr;11:21-29. doi: 10.1016/j.redox.2016.10.013. Epub 2016 Nov 3. Redox Biol. 2017. PMID: 27840283 Free PMC article.
-
L-Ascorbate Biosynthesis Involves Carbon Skeleton Rearrangement in the Nematode Caenorhabditis elegans.Metabolites. 2020 Aug 17;10(8):334. doi: 10.3390/metabo10080334. Metabolites. 2020. PMID: 32824560 Free PMC article.
-
Advances in Antioxidant Applications for Combating 131I Side Effects in Thyroid Cancer Treatment.Toxics. 2023 Jun 13;11(6):529. doi: 10.3390/toxics11060529. Toxics. 2023. PMID: 37368629 Free PMC article. Review.
-
C11orf54 catalyzes L-xylulose formation in human metabolism.Proc Natl Acad Sci U S A. 2025 Aug 5;122(31):e2506597122. doi: 10.1073/pnas.2506597122. Epub 2025 Jul 30. Proc Natl Acad Sci U S A. 2025. PMID: 40737316
References
-
- Buettner GR, Jurkiewicz BA. Catalytic metals, ascorbate and free radicals: combinations to avoid. Radiat. Res. 1996;145:532–541. - PubMed
-
- Gao Q, Zhang L. Ultraviolet-B-induced oxidative stress and antioxidant defense system responses in ascorbate-deficient vtc1 mutants of Arabidopsis thaliana . J. Plant Physiol. 2008;165:138–148. - PubMed
-
- Barth C, De Tullio M, Conklin PL. The role of ascorbic acid in the control of flowering time and the onset of senescence. J. Exp. Bot. 2006;57:1657–1665. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

