Reaction design, discovery, and development as a foundation to function-oriented synthesis
- PMID: 25668752
- PMCID: PMC4363280
- DOI: 10.1021/ar500408e
Reaction design, discovery, and development as a foundation to function-oriented synthesis
Abstract
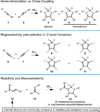
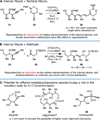
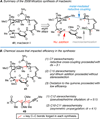
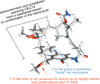
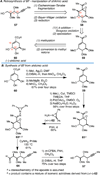
Convergent C-C bond-forming reactions define the fabric of organic synthesis and, when applied in complex molecule synthesis, can have a profound impact on efficiency by decreasing the longest linear sequence of transformations required to convert simple starting materials to complex targets. Despite their well-appreciated strategic significance, campaigns in natural product synthesis typically embrace only a small suite of reactivity to achieve such bond construction (i.e., nucleophilic addition to polarized π-bonds, nucleophilic substitution, cycloaddition, and metal-catalyzed "cross-coupling"), therefore limiting the sites at which convergent coupling chemistry can be strategically employed. In our opinion, it is far too often that triumphs in the field are defined by chemical sequences that do not address the challenges associated with discovery, development, and production of natural product-inspired agents. We speculated that advancing an area of chemical reactivity not represented in the few well-established strategies for convergent C-C bond formation may lead to powerful new retrosynthetic relationships that could simplify approaches to the syntheses of a variety of different classes of natural products. Our studies ultimately embraced the pursuit of strategies to control the course of metallacycle-mediated "cross-coupling" between substrates containing sites of simple π-unsaturation (ubiquitous functionality in organic chemistry including alkenes, alkynes, allenes, aldehydes, and imines, among others). In just eight years since our initial publication in this area, we have defined over 20 stereoselective intermolecular C-C bond-forming reactions that provide access to structural motifs of relevance for the synthesis of polyketides, fatty acids, alkaloids, and terpenes, while doing so in a direct and stereoselective fashion. These achievements continue to serve as the foundation of my group's activity in natural product and function-oriented synthesis, where our achievements in reaction development are challenged in the context of complex targets. Among our early efforts, we achieved the most concise synthesis of a benzoquinone ansamycin ever described (macbecin I), and moved beyond this achievement to explore the role of our chemistry in function-oriented synthesis targeting the discovery of natural product-inspired Hsp90 inhibitors. These later efforts have led to the discovery of a uniquely selective benzoquinone ansamycin-inspired Hsp90 inhibitor that lacks the problematic quinone present in the natural series. This achievement was made possible by a concise chemical synthesis pathway that had at its core the application of metallacycle-mediated cross-coupling chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
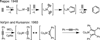







References
-
-
Oestreich M, editor. The Mizoroki-Heck Reaction. New York: Wiley; 2009. p. 608. For recent new directions, see: Mei T-S, Werner EW, Burckle AJ, Sigman MS. Enantio-selective Redox-Relay Oxidative Heck Arylations of Acyclic Alkenyl Alcohols Using Boronic Acids. J. Am. Chem. Soc. 2013;135:6830–6833.
-
-
- Grela K, editor. Olefin Metathesis: Theory and Practice. Hoboken, New Jersey: Wiley; 2014. p. 608.
-
- Reppe W, Schweckendick WJ. Cyclisierende Polymerisation von Acetylen. III Benzol, Benzolderivate und hydroaromatische Verbindungen. Justus Liebigs Ann. Chem. 1948;560:104–116.
- Vol’pin ME, Dubovitskii VA, Nogina OV, Kursanov DN. Titanocene Compounds with Tolanoe. Dokl. Akad. Nauk SSSR. 1963;151:1100.
-
-
For recent reviews, see: Reichard HA, McLaughlin M, Chen MZ, Micalizio GC. Regioselective Reductive Cross-Coupling Reactions of Unsymmetrical Alkynes. Eur. J. Org. Chem. 2010:391–409. Reichard HA, Micalizio GC. Metallacycle-mediated cross-coupling with substituted and electronically unactivated alkenes. Chem. Sci. 2011;2:573–589.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

