Nanogel-based pneumococcal surface protein A nasal vaccine induces microRNA-associated Th17 cell responses with neutralizing antibodies against Streptococcus pneumoniae in macaques
- PMID: 25669148
- PMCID: PMC4762909
- DOI: 10.1038/mi.2015.5
Nanogel-based pneumococcal surface protein A nasal vaccine induces microRNA-associated Th17 cell responses with neutralizing antibodies against Streptococcus pneumoniae in macaques
Abstract
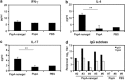
We previously established a nanosized nasal vaccine delivery system by using a cationic cholesteryl group-bearing pullulan nanogel (cCHP nanogel), which is a universal protein-based antigen-delivery vehicle for adjuvant-free nasal vaccination. In the present study, we examined the central nervous system safety and efficacy of nasal vaccination with our developed cCHP nanogel containing pneumococcal surface protein A (PspA-nanogel) against pneumococcal infection in nonhuman primates. When [(18)F]-labeled PspA-nanogel was nasally administered to a rhesus macaque (Macaca mulatta), longer-term retention of PspA was noted in the nasal cavity when compared with administration of PspA alone. Of importance, no deposition of [(18)F]-PspA was seen in the olfactory bulbs or brain. Nasal PspA-nanogel vaccination effectively induced PspA-specific serum IgG with protective activity and mucosal secretory IgA (SIgA) Ab responses in cynomolgus macaques (Macaca fascicularis). Nasal PspA-nanogel-induced immune responses were mediated through T-helper (Th) 2 and Th17 cytokine responses concomitantly with marked increases in the levels of miR-181a and miR-326 in the serum and respiratory tract tissues, respectively, of the macaques. These results demonstrate that nasal PspA-nanogel vaccination is a safe and effective strategy for the development of a nasal vaccine for the prevention of pneumonia in humans.
Figures




Similar articles
-
Nanogel-based PspA intranasal vaccine prevents invasive disease and nasal colonization by Streptococcus pneumoniae.Infect Immun. 2013 May;81(5):1625-34. doi: 10.1128/IAI.00240-13. Epub 2013 Mar 4. Infect Immun. 2013. PMID: 23460513 Free PMC article.
-
A nanogel-based trivalent PspA nasal vaccine protects macaques from intratracheal challenge with pneumococci.Vaccine. 2021 Jun 8;39(25):3353-3364. doi: 10.1016/j.vaccine.2021.04.069. Epub 2021 May 17. Vaccine. 2021. PMID: 34016473
-
Characterization and Specification of a Trivalent Protein-Based Pneumococcal Vaccine Formulation Using an Adjuvant-Free Nanogel Nasal Delivery System.Mol Pharm. 2021 Apr 5;18(4):1582-1592. doi: 10.1021/acs.molpharmaceut.0c01003. Epub 2021 Feb 23. Mol Pharm. 2021. PMID: 33621107
-
Cationic pullulan nanogel as a safe and effective nasal vaccine delivery system for respiratory infectious diseases.Hum Vaccin Immunother. 2018;14(9):2189-2193. doi: 10.1080/21645515.2018.1461298. Epub 2018 Jul 19. Hum Vaccin Immunother. 2018. PMID: 29624474 Free PMC article. Review.
-
Dendritic cell-targeting DNA-based nasal adjuvants for protective mucosal immunity to Streptococcus pneumoniae.Microbiol Immunol. 2017 Jun;61(6):195-205. doi: 10.1111/1348-0421.12487. Microbiol Immunol. 2017. PMID: 28463465 Free PMC article. Review.
Cited by
-
Mucosal vaccines: wisdom from now and then.Int Immunol. 2021 Nov 25;33(12):767-774. doi: 10.1093/intimm/dxab056. Int Immunol. 2021. PMID: 34436595 Free PMC article. Review.
-
Polymeric nanoparticle vaccines to combat emerging and pandemic threats.Biomaterials. 2021 Jan;268:120597. doi: 10.1016/j.biomaterials.2020.120597. Epub 2020 Dec 10. Biomaterials. 2021. PMID: 33360074 Free PMC article. Review.
-
Development of Nasal Vaccines and the Associated Challenges.Pharmaceutics. 2022 Sep 20;14(10):1983. doi: 10.3390/pharmaceutics14101983. Pharmaceutics. 2022. PMID: 36297419 Free PMC article. Review.
-
The Well-Developed Mucosal Immune Systems of Birds and Mammals Allow for Similar Approaches of Mucosal Vaccination in Both Types of Animals.Front Nutr. 2018 Jul 12;5:60. doi: 10.3389/fnut.2018.00060. eCollection 2018. Front Nutr. 2018. PMID: 30050906 Free PMC article. Review.
-
Research progress of self-assembled nanogel and hybrid hydrogel systems based on pullulan derivatives.Drug Deliv. 2018 Nov;25(1):278-292. doi: 10.1080/10717544.2018.1425776. Drug Deliv. 2018. PMID: 29334800 Free PMC article. Review.
References
-
- 2Nuorti, J.P. & Whitney, C.G. Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 59, 1–18 (2010). - PubMed
-
- 3Oosterhuis-Kafeja, F., Beutels, P. & Van Damme, P. Immunogenicity, efficacy, safety and effectiveness of pneumococcal conjugate vaccines (1998-2006). Vaccine 25, 2194–2212 (2007). - PubMed
-
- 4Dagan, R. et al. Reduction of nasopharyngeal carriage of Streptococcus pneumoniae after administration of a 9-valent pneumococcal conjugate vaccine to toddlers attending day care centers. J. Infect. Dis. 185, 927–936 (2002). - PubMed
-
- 5Dagan, R. et al. Comparative immunogenicity and efficacy of 13-valent and 7-valent pneumococcal conjugate vaccines in reducing nasopharyngeal colonization: a randomized double-blind trial. Clin. Infect. Dis. 57, 952–962 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

