Gliovascular disruption and cognitive deficits in a mouse model with features of small vessel disease
- PMID: 25669904
- PMCID: PMC4640247
- DOI: 10.1038/jcbfm.2015.12
Gliovascular disruption and cognitive deficits in a mouse model with features of small vessel disease
Abstract
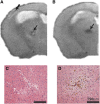
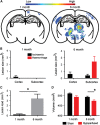
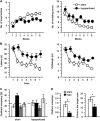
Cerebral small vessel disease (SVD) is a major cause of age-related cognitive impairment and dementia. The pathophysiology of SVD is not well understood and is hampered by a limited range of relevant animal models. Here, we describe gliovascular alterations and cognitive deficits in a mouse model of sustained cerebral hypoperfusion with features of SVD (microinfarcts, hemorrhage, white matter disruption) induced by bilateral common carotid stenosis. Multiple features of SVD were determined on T2-weighted and diffusion-tensor magnetic resonance imaging scans and confirmed by pathologic assessment. These features, which were absent in sham controls, included multiple T2-hyperintense infarcts and T2-hypointense hemosiderin-like regions in subcortical nuclei plus increased cerebral atrophy compared with controls. Fractional anisotropy was also significantly reduced in several white matter structures including the corpus callosum. Investigation of gliovascular changes revealed a marked increase in microvessel diameter, vascular wall disruption, fibrinoid necrosis, hemorrhage, and blood-brain barrier alterations. Widespread reactive gliosis, including displacement of the astrocytic water channel, aquaporin 4, was observed. Hypoperfused mice also demonstrated deficits in spatial working and reference memory tasks. Overall, gliovascular disruption is a prominent feature of this mouse, which could provide a useful model for early-phase testing of potential SVD treatment strategies.
Figures



Similar articles
-
White matter lesions, cerebral inflammation and cognitive function in a mouse model of cerebral hypoperfusion.Brain Res. 2019 May 15;1711:193-201. doi: 10.1016/j.brainres.2019.01.017. Epub 2019 Jan 16. Brain Res. 2019. PMID: 30659829
-
White matter degeneration in vascular and other ageing-related dementias.J Neurochem. 2018 Mar;144(5):617-633. doi: 10.1111/jnc.14271. Epub 2018 Jan 9. J Neurochem. 2018. PMID: 29210074 Review.
-
Differential relationships between apathy and depression with white matter microstructural changes and functional outcomes.Brain. 2015 Dec;138(Pt 12):3803-15. doi: 10.1093/brain/awv304. Epub 2015 Oct 21. Brain. 2015. PMID: 26490330 Free PMC article.
-
Fractal dimension of cerebral white matter: A consistent feature for prediction of the cognitive performance in patients with small vessel disease and mild cognitive impairment.Neuroimage Clin. 2019;24:101990. doi: 10.1016/j.nicl.2019.101990. Epub 2019 Aug 22. Neuroimage Clin. 2019. PMID: 31491677 Free PMC article.
-
Cerebral small vessel disease: Recent advances and future directions.Int J Stroke. 2023 Jan;18(1):4-14. doi: 10.1177/17474930221144911. Int J Stroke. 2023. PMID: 36575578 Free PMC article. Review.
Cited by
-
What type of cell death occurs in chronic cerebral hypoperfusion? A review focusing on pyroptosis and its potential therapeutic implications.Front Cell Neurosci. 2023 Mar 2;17:1073511. doi: 10.3389/fncel.2023.1073511. eCollection 2023. Front Cell Neurosci. 2023. PMID: 36937182 Free PMC article. Review.
-
Endothelial damage, vascular bagging and remodeling of the microvascular bed in human microangiopathy with deep white matter lesions.Acta Neuropathol Commun. 2018 Nov 23;6(1):128. doi: 10.1186/s40478-018-0632-z. Acta Neuropathol Commun. 2018. PMID: 30470258 Free PMC article.
-
Small Vessel Disease.Front Neurol. 2019 Sep 24;10:1020. doi: 10.3389/fneur.2019.01020. eCollection 2019. Front Neurol. 2019. PMID: 31616367 Free PMC article. Review.
-
Does pathology of small venules contribute to cerebral microinfarcts and dementia?J Neurochem. 2018 Mar;144(5):517-526. doi: 10.1111/jnc.14228. Epub 2017 Nov 7. J Neurochem. 2018. PMID: 28950410 Free PMC article. Review.
-
Rodent Models of Cerebral Microinfarct and Microhemorrhage.Stroke. 2018 Mar;49(3):803-810. doi: 10.1161/STROKEAHA.117.016995. Epub 2018 Feb 19. Stroke. 2018. PMID: 29459393 Free PMC article. Review. No abstract available.
References
-
- 1van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan Study. Stroke 2008; 39: 2712–2719. - PubMed
-
- 3Kalaria RN. Cerebrovascular disease and mechanisms of cognitive impairment: evidence from clinicopathological studies in humans. Stroke 2012; 43: 2526–2534. - PubMed
-
- 4Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010; 9: 689–701. - PubMed
-
- 5Fernando MS, Ince PG. Vascular pathologies and cognition in a population-based cohort of elderly people. J Neurol Sci 2004; 226: 13–17. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

