Intracranial pressure elevation reduces flow through collateral vessels and the penetrating arterioles they supply. A possible explanation for 'collateral failure' and infarct expansion after ischemic stroke
- PMID: 25669909
- PMCID: PMC4420869
- DOI: 10.1038/jcbfm.2015.2
Intracranial pressure elevation reduces flow through collateral vessels and the penetrating arterioles they supply. A possible explanation for 'collateral failure' and infarct expansion after ischemic stroke
Abstract
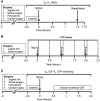
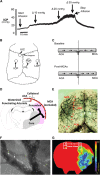
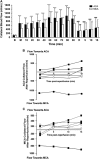
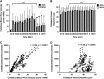
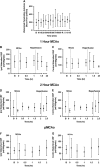
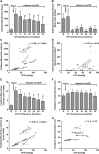
Recent human imaging studies indicate that reduced blood flow through pial collateral vessels ('collateral failure') is associated with late infarct expansion despite stable arterial occlusion. The cause for 'collateral failure' is unknown. We recently showed that intracranial pressure (ICP) rises dramatically but transiently 24 hours after even minor experimental stroke. We hypothesized that ICP elevation would reduce collateral blood flow. First, we investigated the regulation of flow through collateral vessels and the penetrating arterioles arising from them during stroke reperfusion. Wistar rats were subjected to intraluminal middle cerebral artery (MCA) occlusion (MCAo). Individual pial collateral and associated penetrating arteriole blood flow was quantified using fluorescent microspheres. Baseline bidirectional flow changed to MCA-directed flow and increased by >450% immediately after MCAo. Collateral diameter changed minimally. Second, we determined the effect of ICP elevation on collateral and watershed penetrating arteriole flow. Intracranial pressure was artificially raised in stepwise increments during MCAo. The ICP increase was strongly correlated with collateral and penetrating arteriole flow reductions. Changes in collateral flow post-stroke appear to be primarily driven by the pressure drop across the collateral vessel, not vessel diameter. The ICP elevation reduces cerebral perfusion pressure and collateral flow, and is the possible explanation for 'collateral failure' in stroke-in-progression.
Figures
References
-
- Davalos A, Castillo J.Progressing strokeFisher M, Bogousslavsky J. Current Review of Cerebrovascular Disease Philadelphia: Springer; 2001pp 169–181.
-
- Ali LK, Saver JL. The ischemic stroke patient who worsens: new assessment and management approaches. Rev Neurol Dis. 2007;4:85–91. - PubMed
-
- Coutts SB, Modi J, Patel SK, Aram H, Demchuk AM, Goyal M, et al. What causes disability after transient ischemic attack and minor stroke?: Results from the CT and MRI in the Triage of TIA and minor Cerebrovascular Events to Identify High Risk Patients (CATCH) Study. Stroke. 2012;43:3018–3022. - PubMed
-
- Caplan LR. Treatment of "progressive" stroke. Stroke. 1991;22:694–695. - PubMed
-
- Roden-Jullig A, Britton M. Effectiveness of heparin treatment for progressing ischaemic stroke: before and after study. J Intern Med. 2000;248:287–291. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

