The effects of capillary transit time heterogeneity (CTH) on brain oxygenation
- PMID: 25669911
- PMCID: PMC4420854
- DOI: 10.1038/jcbfm.2014.254
The effects of capillary transit time heterogeneity (CTH) on brain oxygenation
Abstract
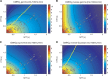
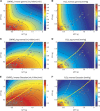
We recently extended the classic flow-diffusion equation, which relates blood flow to tissue oxygenation, to take capillary transit time heterogeneity (CTH) into account. Realizing that cerebral oxygen availability depends on both cerebral blood flow (CBF) and capillary flow patterns, we have speculated that CTH may be actively regulated and that changes in the capillary morphology and function, as well as in blood rheology, may be involved in the pathogenesis of conditions such as dementia and ischemia-reperfusion injury. The first extended flow-diffusion equation involved simplifying assumptions which may not hold in tissue. Here, we explicitly incorporate the effects of oxygen metabolism on tissue oxygen tension and extraction efficacy, and assess the extent to which the type of capillary transit time distribution affects the overall effects of CTH on flow-metabolism coupling reported earlier. After incorporating tissue oxygen metabolism, our model predicts changes in oxygen consumption and tissue oxygen tension during functional activation in accordance with literature reports. We find that, for large CTH values, a blood flow increase fails to cause significant improvements in oxygen delivery, and can even decrease it; a condition of malignant CTH. These results are found to be largely insensitive to the choice of the transit time distribution.
Figures




References
-
- Derdeyn CP, Videen TO, Yundt KD, Fritsch SM, Carpenter DA, Grubb RL, et al. Variability of cerebral blood volume and oxygen extraction: stages of cerebral haemodynamic impairment revisited. Brain J Neurol. 2002;125 (Pt 3:595–607. - PubMed
-
- Renkin EM. B. W. Zweifach Award lecture. Regulation of the microcirculation. Microvasc Res. 1985;30:251–263. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

