The heparan sulfate mimetic PG545 interferes with Wnt/β-catenin signaling and significantly suppresses pancreatic tumorigenesis alone and in combination with gemcitabine
- PMID: 25669977
- PMCID: PMC4467129
- DOI: 10.18632/oncotarget.3214
The heparan sulfate mimetic PG545 interferes with Wnt/β-catenin signaling and significantly suppresses pancreatic tumorigenesis alone and in combination with gemcitabine
Erratum in
-
Correction: The heparan sulfate mimetic PG545 interferes with Wnt/β-catenin signaling and significantly suppresses pancreatic tumorigenesis alone and in combination with gemcitabine.Oncotarget. 2019 Sep 24;10(54):5669. doi: 10.18632/oncotarget.27216. eCollection 2019 Sep 24. Oncotarget. 2019. PMID: 31608142 Free PMC article.
Abstract
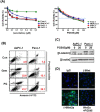
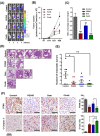
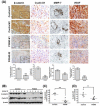
The heparan sulfate mimetic PG545 has been shown to exert anti-angiogenic and anti-metastatic activity in vitro and in vivo cancer models. Although much of this activity has been attributed to inhibition of heparanase and heparan sulfate-binding growth factors, it was hypothesized that PG545 may additionally disrupt Wnt signaling, an important pathway underlying the malignancy of pancreatic cancer. We show that PG545, by directly interacting with Wnt3a and Wnt7a, inhibits Wnt/β-catenin signaling leading to inhibition of proliferation in pancreatic tumor cell lines. Additionally, we demonstrate for the first time that the combination of PG545 with gemcitabine has strong synergistic effects on viability, motility and apoptosis induction in several pancreatic cell lines. In an orthotopic xenograft mouse model, combination of PG545 with gemcitabine efficiently inhibited tumor growth and metastasis compared to single treatment alone. Also, PG545 treatment alone decreased the levels of β-catenin and its downstream targets, cyclin D1, MMP-7 and VEGF which is consistent with our in vitro data. Collectively, our findings suggest that PG545 exerts anti-tumor activity by disrupting Wnt/β-catenin signaling and combination with gemcitabine should be considered as a novel therapeutic strategy for pancreatic cancer treatment.
Keywords: PG545; Wnt/β-catenin; gemcitabine; heparan sulfate mimetic; pancreatic cancer.
Conflict of interest statement
The authors disclose no potential conflicts of interest with the exception of EH and KD who are full time employees of Progen Pharmaceuticals Ltd.
Figures



References
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. - PubMed
-
- Di Marco M, Di Cicilia R, Macchini M, Nobili E, Vecchiarelli S, Brandi G, Biasco G. Metastatic pancreatic cancer: is gemcitabine still the best standard treatment? (Review) Oncol Rep. 2010;23(5):1183–1192. - PubMed
-
- Gui S, Yuan G, Wang L, Zhou L, Xue Y, Yu Y, Zhang J, Zhang M, Yang Y, Wang DW. Wnt3a regulates proliferation, apoptosis and function of pancreatic NIT-1 beta cells via activation of IRS2/PI3K signaling. J Cell Biochem. 2013;114(7):1488–1497. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

