Rapid changes in gene expression direct rapid shifts in intestinal form and function in the Burmese python after feeding
- PMID: 25670730
- PMCID: PMC4421790
- DOI: 10.1152/physiolgenomics.00131.2014
Rapid changes in gene expression direct rapid shifts in intestinal form and function in the Burmese python after feeding
Abstract
Snakes provide a unique and valuable model system for studying the extremes of physiological remodeling because of the ability of some species to rapidly upregulate organ form and function upon feeding. The predominant model species used to study such extreme responses has been the Burmese python because of the extreme nature of postfeeding response in this species. We analyzed the Burmese python intestine across a time series, before, during, and after feeding to understand the patterns and timing of changes in gene expression and their relationship to changes in intestinal form and function upon feeding. Our results indicate that >2,000 genes show significant changes in expression in the small intestine following feeding, including genes involved in intestinal morphology and function (e.g., hydrolases, microvillus proteins, trafficking and transport proteins), as well as genes involved in cell division and apoptosis. Extensive changes in gene expression occur surprisingly rapidly, within the first 6 h of feeding, coincide with changes in intestinal morphology, and effectively return to prefeeding levels within 10 days. Collectively, our results provide an unprecedented portrait of parallel changes in gene expression and intestinal morphology and physiology on a scale that is extreme both in the magnitude of changes, as well as in the incredibly short time frame of these changes, with up- and downregulation of expression and function occurring in the span of 10 days. Our results also identify conserved vertebrate signaling pathways that modulate these responses, which may suggest pathways for therapeutic modulation of intestinal function in humans.
Keywords: RNA-Seq; hyperplasia; organ remodeling; physiological remodeling; small intestine.
Copyright © 2015 the American Physiological Society.
Figures
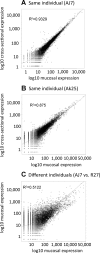




Similar articles
-
Digestive physiology of the Burmese python: broad regulation of integrated performance.J Exp Biol. 2008 Dec;211(Pt 24):3767-74. doi: 10.1242/jeb.023754. J Exp Biol. 2008. PMID: 19043049
-
Growth and stress response mechanisms underlying post-feeding regenerative organ growth in the Burmese python.BMC Genomics. 2017 May 2;18(1):338. doi: 10.1186/s12864-017-3743-1. BMC Genomics. 2017. PMID: 28464824 Free PMC article.
-
Selected regulation of gastrointestinal acid-base secretion and tissue metabolism for the diamondback water snake and Burmese python.J Exp Biol. 2012 Jan 1;215(Pt 1):185-96. doi: 10.1242/jeb.056218. J Exp Biol. 2012. PMID: 22162867
-
Utility of the burmese Python as a model for studying plasticity of extreme physiological systems.J Muscle Res Cell Motil. 2023 Jun;44(2):95-106. doi: 10.1007/s10974-022-09632-2. Epub 2022 Nov 1. J Muscle Res Cell Motil. 2023. PMID: 36316565 Free PMC article. Review.
-
Effects of digestive status on the reptilian gut.Comp Biochem Physiol A Mol Integr Physiol. 2002 Nov;133(3):499-518. doi: 10.1016/s1095-6433(02)00257-x. Comp Biochem Physiol A Mol Integr Physiol. 2002. PMID: 12443909 Review.
Cited by
-
Single-cell resolution of intestinal regeneration in pythons without crypts illuminates conserved vertebrate regenerative mechanisms.Proc Natl Acad Sci U S A. 2024 Oct 22;121(43):e2405463121. doi: 10.1073/pnas.2405463121. Epub 2024 Oct 18. Proc Natl Acad Sci U S A. 2024. PMID: 39423244 Free PMC article.
-
Multi-species comparisons of snakes identify coordinated signalling networks underlying post-feeding intestinal regeneration.Proc Biol Sci. 2019 Jul 10;286(1906):20190910. doi: 10.1098/rspb.2019.0910. Epub 2019 Jul 10. Proc Biol Sci. 2019. PMID: 31288694 Free PMC article.
-
Drosophila activins adapt gut size to food intake and promote regenerative growth.Nat Commun. 2024 Jan 4;15(1):273. doi: 10.1038/s41467-023-44553-9. Nat Commun. 2024. PMID: 38177201 Free PMC article.
-
Identification of an integrated stress and growth response signaling switch that directs vertebrate intestinal regeneration.BMC Genomics. 2022 Jan 4;23(1):6. doi: 10.1186/s12864-021-08226-5. BMC Genomics. 2022. PMID: 34983392 Free PMC article.
-
Leveraging Comparative Genomics to Identify and Functionally Characterize Genes Associated with Sperm Phenotypes in Python bivittatus (Burmese Python).Genet Res Int. 2016;2016:7505268. doi: 10.1155/2016/7505268. Epub 2016 Apr 20. Genet Res Int. 2016. PMID: 27200191 Free PMC article.
References
-
- Adade CM, Cons BL, Melo PA, Souto-Padron T. Effect of Crotalus viridis viridis snake venom on the ultrastructure and intracellular survival of Trypanosoma cruzi. Parasitology 138: 46–58, 2011. - PubMed
-
- Andersen JB, Rourke BC, Caiozzo VJ, Bennett AF, Hicks JW. Physiology: postprandial cardiac hypertrophy in pythons. Nature 434: 37–38, 2005. - PubMed
-
- Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 369: 1641–1657, 2007. - PubMed
-
- Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell 103: 311–320, 2000. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

