Sphingomyelin metabolism is involved in the differentiation of MDCK cells induced by environmental hypertonicity
- PMID: 25670801
- PMCID: PMC4373737
- DOI: 10.1194/jlr.M050781
Sphingomyelin metabolism is involved in the differentiation of MDCK cells induced by environmental hypertonicity
Abstract
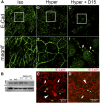
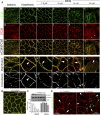
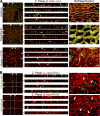
Sphingolipids (SLs) are relevant lipid components of eukaryotic cells. Besides regulating various cellular processes, SLs provide the structural framework for plasma membrane organization. Particularly, SM is associated with detergent-resistant microdomains. We have previously shown that the adherens junction (AJ) complex, the relevant cell-cell adhesion structure involved in cell differentiation and tissue organization, is located in an SM-rich membrane lipid domain. We have also demonstrated that under hypertonic conditions, Madin-Darby canine kidney (MDCK) cells acquire a differentiated phenotype with changes in SL metabolism. For these reasons, we decided to evaluate whether SM metabolism is involved in the acquisition of the differentiated phenotype of MDCK cells. We found that SM synthesis mediated by SM synthase 1 is involved in hypertonicity-induced formation of mature AJs, necessary for correct epithelial cell differentiation. Inhibition of SM synthesis impaired the acquisition of mature AJs, evoking a disintegration-like process reflected by the dissipation of E-cadherin and β- and α-catenins from the AJ complex. As a consequence, MDCK cells did not develop the hypertonicity-induced differentiated epithelial cell phenotype.
Keywords: Madin-Darby canine kidney cells; renal epithelial cells; sphingomyelin synthase.
Copyright © 2015 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Ogretmen B., Hannun Y. A. 2004. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat. Rev. Cancer. 4: 604–616. - PubMed
-
- van Echten G., Birk R., Brenner-Weiss G., Schmidt R. R., Sandhoff K. 1990. Modulation of sphingolipid biosynthesis in primary cultured neurons by long chain bases. J. Biol. Chem. 265: 9333–9339. - PubMed
-
- Merrill A. H., Jones D. D. 1990. An update of the enzymology and regulation of sphingomyelin metabolism. Biochim. Biophys. Acta. 1044: 1–12. - PubMed
-
- Slotte J. P. 2013. Biological functions of sphingomyelins. Prog. Lipid Res. 52: 424–437. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

