Ularitide for the treatment of acute decompensated heart failure: from preclinical to clinical studies
- PMID: 25670819
- PMCID: PMC4368857
- DOI: 10.1093/eurheartj/ehu484
Ularitide for the treatment of acute decompensated heart failure: from preclinical to clinical studies
Abstract
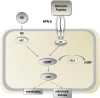
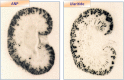
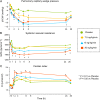
The short- and long-term morbidity and mortality in acute heart failure is still unacceptably high. There is an unmet need for new therapy options with new drugs with a new mode of action. One of the drugs currently in clinical testing in Phase III is ularitide, which is the chemically synthesized form of the human natriuretic peptide urodilatin. Urodilatin is produced in humans by differential processing of pro-atrial natriuretic peptide in distal renal tubule cells. Physiologically, urodilatin appears to be the natriuretic peptide involved in sodium homeostasis. Ularitide exerts its pharmacological actions such as vasodilation, diuresis, and natriuresis through the natriuretic peptide receptor/particulate guanylate cyclase/cyclic guanosine monophosphate pathway. In animal models of heart failure as well as Phase I and II clinical studies in heart failure patients, ularitide demonstrated beneficial effects such as symptom relief and vasodilation, while still preserving renal function. Subsequently, the pivotal acute decompensated heart failure (ADHF) Phase III study, called TRUE-AHF, was started with the objectives to evaluate the effects of ularitide infusion on the clinical status and cardiovascular mortality of patients with ADHF compared with placebo. This review summarizes preclinical and clinical data supporting the potential use of ularitide in the treatment of ADHF.
Keywords: Acute decompensated heart failure; Diuresis; Guanylate cyclase; Natriuresis; Ularitide; Vasodilation.
© The Author 2015. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures



Similar articles
-
Ularitide: a natriuretic peptide candidate for the treatment of acutely decompensated heart failure.Future Cardiol. 2015 Sep;11(5):531-46. doi: 10.2217/fca.15.53. Epub 2015 Aug 17. Future Cardiol. 2015. PMID: 26278236 Review.
-
Therapeutic use of the natriuretic peptide ularitide in acute renal failure.Ren Fail. 1999 Jan;21(1):85-100. doi: 10.3109/08860229909066972. Ren Fail. 1999. PMID: 10048120 Clinical Trial.
-
The renal paracrine peptide system--possible urologic implications of urodilatin.World J Urol. 1996;14(6):375-9. doi: 10.1007/BF00183118. World J Urol. 1996. PMID: 8986039 Review.
-
Role of guanylate cyclase modulators in decompensated heart failure.Heart Fail Rev. 2009 Dec;14(4):309-19. doi: 10.1007/s10741-009-9149-7. Heart Fail Rev. 2009. PMID: 19568931 Review.
-
Renal effects of ularitide in patients with decompensated heart failure.Am Heart J. 2008 Jun;155(6):1012.e1-8. doi: 10.1016/j.ahj.2008.02.011. Am Heart J. 2008. PMID: 18513512 Clinical Trial.
Cited by
-
Urinary urodilatin levels in patients with renal salt wasting syndrome as a possible new diagnostic marker. A pilot study.Front Pharmacol. 2025 Jun 11;16:1579699. doi: 10.3389/fphar.2025.1579699. eCollection 2025. Front Pharmacol. 2025. PMID: 40567378 Free PMC article.
-
Atrial natriuretic peptide in cardiovascular biology and disease (NPPA).Gene. 2015 Sep 10;569(1):1-6. doi: 10.1016/j.gene.2015.06.029. Epub 2015 Jun 12. Gene. 2015. PMID: 26074089 Free PMC article. Review.
-
Natriuretic Peptides in the Regulation of Cardiovascular Physiology and Metabolic Events.J Am Heart Assoc. 2015 Oct 27;4(10):e002423. doi: 10.1161/JAHA.115.002423. J Am Heart Assoc. 2015. PMID: 26508744 Free PMC article. Review. No abstract available.
-
Natriuretic Peptides-New Targets for Neurocontrol of Blood Pressure via Baroreflex Afferent Pathway.Int J Mol Sci. 2022 Nov 7;23(21):13619. doi: 10.3390/ijms232113619. Int J Mol Sci. 2022. PMID: 36362405 Free PMC article.
-
Patterns of dyspnoea onset in patients with acute heart failure: clinical and prognostic implications.ESC Heart Fail. 2019 Feb;6(1):16-26. doi: 10.1002/ehf2.12371. Epub 2018 Nov 13. ESC Heart Fail. 2019. PMID: 30426729 Free PMC article.
References
-
- Forssmann WG, Richter R, Meyer M. The endocrine heart and natriuretic peptides: histochemistry, cell biology, and functional aspects of the renal urodilatin system. Histochem Cell Biol. 1998;110:335–357. - PubMed
-
- Saba SR, Ramirez G, Vesely DL. Immunocytochemical localization of ProANF 1-30, ProANF 31-67, atrial natriuretic factor and urodilatin in the human kidney. Am J Nephrol. 1993;13:85–93. - PubMed
-
- Schulz-Knappe P, Forssmann K, Herbst F, Hock D, Pipkorn R, Forssmann WG. Isolation and structural analysis of “urodilatin”, a new peptide of the cardiodilatin-(ANP)-family, extracted from human urine. Klin Wochenschr. 1988;66:752–759. - PubMed
-
- Goetz KL. Renal natriuretic peptide (urodilatin?) and atriopeptin: evolving concepts. Am J Physiol. 1991;261(6 Pt 2):F921–F932. - PubMed
-
- Nagase M, Katafuchi T, Hirose S, Fujita T. Tissue distribution and localization of natriuretic peptide receptor subtypes in stroke-prone spontaneously hypertensive rats. J Hypertens. 1997;15:1235–1243. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

