Ceftolozane/Tazobactam Plus Metronidazole for Complicated Intra-abdominal Infections in an Era of Multidrug Resistance: Results From a Randomized, Double-Blind, Phase 3 Trial (ASPECT-cIAI)
- PMID: 25670823
- PMCID: PMC4412191
- DOI: 10.1093/cid/civ097
Ceftolozane/Tazobactam Plus Metronidazole for Complicated Intra-abdominal Infections in an Era of Multidrug Resistance: Results From a Randomized, Double-Blind, Phase 3 Trial (ASPECT-cIAI)
Abstract
Background: Increasing antimicrobial resistance among pathogens causing complicated intra-abdominal infections (cIAIs) supports the development of new antimicrobials. Ceftolozane/tazobactam, a novel antimicrobial therapy, is active against multidrug-resistant Pseudomonas aeruginosa and most extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae.
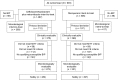
Methods: ASPECT-cIAI (Assessment of the Safety Profile and Efficacy of Ceftolozane/Tazobactam in Complicated Intra-abdominal Infections) was a prospective, randomized, double-blind trial. Hospitalized patients with cIAI received either ceftolozane/tazobactam (1.5 g) plus metronidazole (500 mg) every 8 hours or meropenem (1 g) every 8 hours intravenously for 4-14 days. The prospectively defined objectives were to demonstrate statistical noninferiority in clinical cure rates at the test-of-cure visit (24-32 days from start of therapy) in the microbiological intent-to-treat (primary) and microbiologically evaluable (secondary) populations using a noninferiority margin of 10%. Microbiological outcomes and safety were also evaluated.
Results: Ceftolozane/tazobactam plus metronidazole was noninferior to meropenem in the primary (83.0% [323/389] vs 87.3% [364/417]; weighted difference, -4.2%; 95% confidence interval [CI], -8.91 to .54) and secondary (94.2% [259/275] vs 94.7% [304/321]; weighted difference, -1.0%; 95% CI, -4.52 to 2.59) endpoints, meeting the prespecified noninferiority margin. In patients with ESBL-producing Enterobacteriaceae, clinical cure rates were 95.8% (23/24) and 88.5% (23/26) in the ceftolozane/tazobactam plus metronidazole and meropenem groups, respectively, and 100% (13/13) and 72.7% (8/11) in patients with CTX-M-14/15 ESBLs. The frequency of adverse events (AEs) was similar in both treatment groups (44.0% vs 42.7%); the most common AEs in either group were nausea and diarrhea.
Conclusions: Treatment with ceftolozane/tazobactam plus metronidazole was noninferior to meropenem in adult patients with cIAI, including infections caused by multidrug-resistant pathogens.
Clinical trials registration: NCT01445665 and NCT01445678.
Keywords: Enterobacteriaceae; ceftolozane/tazobactam; complicated intra-abdominal infection; gram-negative bacteria; multidrug resistance.
© The Author 2015. Published by Oxford University Press on behalf of the Infectious Diseases Society of America.
Figures

Comment in
-
Noninferiority Doesn't Mean Not Inferior.Clin Infect Dis. 2016 Feb 15;62(4):525-6. doi: 10.1093/cid/civ895. Epub 2015 Oct 20. Clin Infect Dis. 2016. PMID: 26486703 No abstract available.
-
Response to Spellberg and Brass.Clin Infect Dis. 2016 Feb 15;62(4):526. doi: 10.1093/cid/civ896. Epub 2015 Oct 20. Clin Infect Dis. 2016. PMID: 26486708 No abstract available.
References
-
- Barie PS, Hydo LJ, Eachempati SR. Longitudinal outcomes of intra-abdominal infection complicated by critical illness. Surg Infect (Larchmt) 2004; 5:365–73. - PubMed
-
- Gauzit R, Péan Y, Barth X, Mistretta F, Lalaude O. Epidemiology, management, and prognosis of secondary non-postoperative peritonitis: a French prospective observational multicenter study. Surg Infect (Larchmt) 2009; 10:119–27. - PubMed
-
- Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis 2010; 50:133–64. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

