Ubiquitinated sirtuin 1 (SIRT1) function is modulated during DNA damage-induced cell death and survival
- PMID: 25670865
- PMCID: PMC4423681
- DOI: 10.1074/jbc.M114.612796
Ubiquitinated sirtuin 1 (SIRT1) function is modulated during DNA damage-induced cell death and survival
Abstract
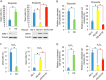
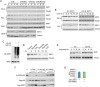
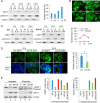
Downstream signaling of physiological and pathological cell responses depends on post-translational modification such as ubiquitination. The mechanisms regulating downstream DNA damage response (DDR) signaling are not completely elucidated. Sirtuin 1 (SIRT1), the founding member of Class III histone deacetylases, regulates multiple steps in DDR and is closely associated with many physiological and pathological processes. However, the role of post-translational modification or ubiquitination of SIRT1 during DDR is unclear. We show that SIRT1 is dynamically and distinctly ubiquitinated in response to DNA damage. SIRT1 was ubiquitinated by the MDM2 E3 ligase in vitro and in vivo. SIRT1 ubiquitination under normal conditions had no effect on its enzymatic activity or rate of degradation; hypo-ubiquitination, however, reduced SIRT1 nuclear localization. Ubiquitination of SIRT1 affected its function in cell death and survival in response to DNA damage. Our results suggest that ubiquitination is required for SIRT1 function during DDR.
Keywords: Deubiquitylation (Deubiquitination); Histone Deacetylase (HDAC); Post-translational Modification (PTM); Sirtuin; Ubiquitylation (Ubiquitination).
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

