Optimization of caseinate-coated simvastatin-zein nanoparticles: improved bioavailability and modified release characteristics
- PMID: 25670883
- PMCID: PMC4315536
- DOI: 10.2147/DDDT.S76194
Optimization of caseinate-coated simvastatin-zein nanoparticles: improved bioavailability and modified release characteristics
Abstract
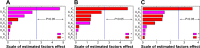
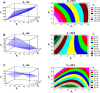
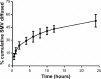
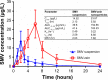
The current study focuses on utilization of the natural biocompatible polymer zein to formulate simvastatin (SMV) nanoparticles coated with caseinate, to improve solubility and hence bioavailability, and in addition, to modify SMV-release characteristics. This formulation can be utilized for oral or possible depot parenteral applications. Fifteen formulations were prepared by liquid-liquid phase separation method, according to the Box-Behnken design, to optimize formulation variables. Sodium caseinate was used as an electrosteric stabilizer. The factors studied were: percentage of SMV in the SMV-zein mixture (X1), ethanol concentration (X2), and caseinate concentration (X3). The selected dependent variables were mean particle size (Y1), SMV encapsulation efficiency (Y2), and cumulative percentage of drug permeated after 1 hour (Y3). The diffusion of SMV from the prepared nanoparticles specified by the design was carried out using an automated Franz diffusion cell apparatus. The optimized SMV-zein formula was investigated for in vivo pharmacokinetic parameters compared with an oral SMV suspension. The optimized nanosized SMV-zein formula showed a 131 nm mean particle size and 89% encapsulation efficiency. In vitro permeation studies displayed delayed permeation characteristics, with about 42% and 85% of SMV cumulative amount released after 12 and 48 hours, respectively. Bioavailability estimation in rats revealed an augmentation in SMV bioavailability from the optimized SMV-zein formulation, by fourfold relative to SMV suspension. Formulation of caseinate-coated SMV-zein nanoparticles improves the pharmacokinetic profile and bioavailability of SMV. Accordingly, improved hypolipidemic activities for longer duration could be achieved. In addition, the reduced dosage rate of SMV-zein nanoparticles improves patient tolerability and compliance.
Keywords: Box–Behnken design; simvastatin; sodium caseinate; zein.
Figures

References
-
- Mishra S, Panda DS, Pradhan M, Hussain I. Preparation and evaluation of ezetimibe nanosuspension. Journal of Advanced Pharmaceutical Research. 2011;2:185–189.
-
- von Briesen H, Ramge P, Kreuter J. Controlled release of antiretroviral drugs. AIDS Rev. 2000;2:31–38.
-
- Baigent C, Keech A, Kearney PM, et al. Cholesterol Treatment Trialists’ (CTT) Collaborators Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. - PubMed
-
- Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol. 2006;48(3):438–445. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

