Conjugation of cell-penetrating peptides with poly(lactic-co-glycolic acid)-polyethylene glycol nanoparticles improves ocular drug delivery
- PMID: 25670897
- PMCID: PMC4315550
- DOI: 10.2147/IJN.S71198
Conjugation of cell-penetrating peptides with poly(lactic-co-glycolic acid)-polyethylene glycol nanoparticles improves ocular drug delivery
Abstract
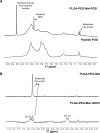
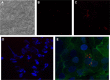
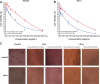
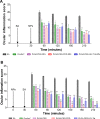
In this work, a peptide for ocular delivery (POD) and human immunodeficiency virus transactivator were conjugated with biodegradable poly(lactic-co-glycolic acid) (PGLA)-polyethylene glycol (PEG)-nanoparticles (NPs) in an attempt to improve ocular drug bioavailability. The NPs were prepared by the solvent displacement method following two different pathways. One involved preparation of PLGA NPs followed by PEG and peptide conjugation (PLGA-NPs-PEG-peptide); the other involved self-assembly of PLGA-PEG and the PLGA-PEG-peptide copolymer followed by NP formulation. The conjugation of the PEG and the peptide was confirmed by a colorimetric test and proton nuclear magnetic resonance spectroscopy. Flurbiprofen was used as an example of an anti-inflammatory drug. The physicochemical properties of the resulting NPs (morphology, in vitro release, cell viability, and ocular tolerance) were studied. In vivo anti-inflammatory efficacy was assessed in rabbit eyes after topical instillation of sodium arachidonate. Of the formulations developed, the PLGA-PEG-POD NPs were the smaller particles and exhibited greater entrapment efficiency and more sustained release. The positive charge on the surface of these NPs, due to the conjugation with the positively charged peptide, facilitated penetration into the corneal epithelium, resulting in more effective prevention of ocular inflammation. The in vitro toxicity of the NPs developed was very low; no ocular irritation in vitro (hen's egg test-chorioallantoic membrane assay) or in vivo (Draize test) was detected. Taken together, these data demonstrate that PLGA-PEG-POD NPs are promising vehicles for ocular drug delivery.
Keywords: anti-inflammatory; controlled release; flurbiprofen; ocular tolerance; peptide for ocular delivery.
Figures


References
-
- Brown RM, Roberts CW. Preoperative and postoperative use of non-steroidal antiinflammatory drugs in cataract surgery. Insight. 1996;21(1):13–16. - PubMed
-
- Bandara BM, Sankaridurg PR, Willcox MD. Non-steroidal anti inflammatory agents decrease bacterial colonisation of contact lenses and prevent adhesion to human corneal epithelial cells. Curr Eye Res. 2004;29(4–5):245–251. - PubMed
-
- Zhang W, Prausnitz MR, Edwards A. Model of transient drug diffusion across cornea. J Control Release. 2004;99(2):241–258. - PubMed
-
- Pignatello R, Bucolo C, Spedalieri G, Maltese A, Puglisi G. Flurbiprofen-loaded acrylate polymer nanosuspensions for ophthalmic application. Biomaterials. 2002;23(15):3247–3255. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

