Release of β-galactosidase from poloxamine/α-cyclodextrin hydrogels
- PMID: 25670982
- PMCID: PMC4311648
- DOI: 10.3762/bjoc.10.330
Release of β-galactosidase from poloxamine/α-cyclodextrin hydrogels
Abstract
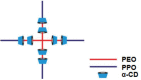
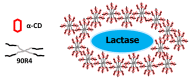
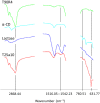
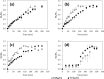
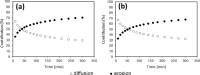
All mammals lose their ability to produce lactase (β-galactosidase), the enzyme that cleaves lactose into galactose and glucose, after weaning. The prevalence of lactase deficiency (LD) spans from 2 to 15% among northern Europeans, to nearly 100% among Asians. Following lactose consumption, people with LD often experience gastrointestinal symptoms such as abdominal pain, bowel distension, cramps and flatulence, or even systemic problems such as headache, loss of concentration and muscle pain. These symptoms vary depending on the amount of lactose ingested, type of food and degree of intolerance. Although those affected can avoid the uptake of dairy products, in doing so, they lose a readily available source of calcium and protein. In this work, gels obtained by complexation of Tetronic 90R4 with α-cyclodextrin loaded with β-galactosidase are proposed as a way to administer the enzyme immediately before or with the lactose-containing meal. Both molecules are biocompatible, can form gels in situ, and show sustained erosion kinetics in aqueous media. The complex was characterized by FTIR that evidenced an inclusion complex between the polyethylene oxide block and α-cyclodextrin. The release profiles of β-galactosidase from two different matrices (gels and tablets) of the in situ hydrogels have been obtained. The influence of the percentage of Tetronic in media of different pH was evaluated. No differences were observed regarding the release rate from the gel matrices at pH 6 (t 50 = 105 min). However, in the case of the tablets, the kinetics were faster and they released a greater amount of 90R4 (25%, t 50 = 40-50 min). Also, the amount of enzyme released was higher for mixtures with 25% Tetronic. Using suitable mathematical models, the corresponding kinetic parameters have been calculated. In all cases, the release data fit quite well to the Peppas-Sahlin model equation, indicating that the release of β-galactosidase is governed by a combination of diffusion and erosion processes. It has been observed that the diffusion mechanism prevails over erosion during the first 50 minutes, followed by continued release of the enzyme due to the disintegration of the matrix.
Keywords: controlled release; cyclodextrins; lactase; polypseudorotaxane; supramolecular gel.
Figures
References
-
- Alliende F G. Gastroenterol Latinoam. 2007;18:152–156.
-
- Hensley A L. [Jul 4;2014 ];The enzyme β-galactosidase: structure and function. Available from: http://chemistry.berea.edu/~biochemistry/2008/ah.
-
- Benevides C. Ingeniería de biocatalizadores y bioprocesos: beta-galactosidasa de Thermus sp., cepa T2. Spain: Universidad Politécnica de Madrid; 2002.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
