Effects of RAMEA-complexed polyunsaturated fatty acids on the response of human dendritic cells to inflammatory signals
- PMID: 25670984
- PMCID: PMC4311633
- DOI: 10.3762/bjoc.10.332
Effects of RAMEA-complexed polyunsaturated fatty acids on the response of human dendritic cells to inflammatory signals
Abstract
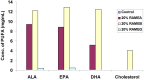
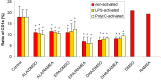
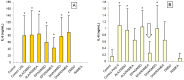
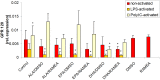
The n-3 fatty acids are not produced by mammals, although they are essential for hormone synthesis and maintenance of cell membrane structure and integrity. They have recently been shown to inhibit inflammatory reactions and also emerged as potential treatment options for inflammatory diseases, such as rheumatoid arthritis, asthma and inflammatory bowel diseases. Dendritic cells (DC) play a central role in the regulation of both innate and adaptive immunity and upon inflammatory signals they produce various soluble factors among them cytokines and chemokines that act as inflammatory or regulatory mediators. In this study we monitored the effects of α-linoleic acid, eicosapentaenoic acid and docosahexaenoic acid solubilized in a dimethyl sulfoxide (DMSO)/ethanol 1:1 mixture or as complexed by randomly methylated α-cyclodextrin (RAMEA) on the inflammatory response of human monocyte-derived dendritic cells (moDC). The use of RAMEA for enhancing aqueous solubility of n-3 fatty acids has the unambiguous advantage over applying RAMEB (the β-cyclodextrin analog), since there is no interaction with cell membrane cholesterol. In vitro differentiated moDC were left untreated or were stimulated by bacterial lipopolysaccharide and polyinosinic:polycytidylic acid, mimicking bacterial and viral infections, respectively. The response of unstimulated and activated moDC to n-3 fatty acid treatment was tested by measuring the cell surface expression of CD1a used as a phenotypic and CD83 as an activation marker of inflammatory moDC differentiation and activation by using flow cytometry. Monocyte-derived DC activation was also monitored by the secretion level of the pro- and anti-inflammatory cytokines IL-1β, TNF-α, IL-6, IL-10 and IL-12, respectively. We found that RAMEA-complexed n-3 fatty acids reduced the expression of CD1a protein in both LPS and Poly(I:C) stimulated moDC significantly, but most efficiently by eicosapentaenic acid, while no significant change in the expression of CD83 protein was observed. The production of IL-6 by LPS-activated moDC was also reduced significantly when eicosapentaenic acid was added as a RAMEA complex as compared to its DMSO-solubilized form or to the other two n-3 fatty acids either complexed or not. Based on these results n-3 fatty acids solubilized by RAMEA provide with a new tool for optimizing the anti-inflammatory effects of n-3 fatty acids exerted on human moDC and mediated through the GP120 receptor without interfering with the cell membrane structure.
Keywords: RAMEA; cell membrane; cell surface markers; cyclodextrin; enzyme immunoassay; flow cytometry; monocyte-derived dendritic cells; proinflammatory cytokines; solubilization.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
