Intestinal anti-inflammatory activity of Sasa quelpaertensis leaf extract by suppressing lipopolysaccharide-stimulated inflammatory mediators in intestinal epithelial Caco-2 cells co-cultured with RAW 264.7 macrophage cells
- PMID: 25671061
- PMCID: PMC4317476
- DOI: 10.4162/nrp.2015.9.1.3
Intestinal anti-inflammatory activity of Sasa quelpaertensis leaf extract by suppressing lipopolysaccharide-stimulated inflammatory mediators in intestinal epithelial Caco-2 cells co-cultured with RAW 264.7 macrophage cells
Abstract
Background/objectives: Inflammatory bowel disease (IBD), including Crohn's disease and ulcerative colitis, involves chronic inflammation of the gastrointestinal tract. Previously, Sasa quelpaertensis leaves have been shown to mediate anti-inflammation and anti-cancer effects, although it remains unclear whether Sasa leaves are able to attenuate inflammation-related intestinal diseases. Therefore, the aim of this study was to investigate the anti-inflammatory effects of Sasa quelpaertensis leaf extract (SQE) using an in vitro co-culture model of the intestinal epithelial environment.
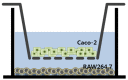
Materials/methods: An in vitro co-culture system was established that consisted of intestinal epithelial Caco-2 cells and RAW 264.7 macrophages. Treatment with lipopolysaccharide (LPS) was used to induce inflammation.
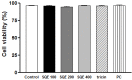
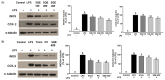
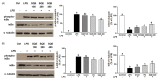
Results: Treatment with SQE significantly suppressed the secretion of LPS-induced nitric oxide (NO), prostaglandin E2 (PGE2), IL-6, and IL-1β in co-cultured RAW 264.7 macrophages. In addition, expressions of inducible nitric oxide synthase (iNOS), cyclooxygenase (COX)-2, and tumor necrosis factor (TNF)-α were down-regulated in response to inhibition of IκBα phosphorylation by SQE. Compared with two bioactive compounds that have previously been identified in SQE, tricin and P-coumaric acid, SQE exhibited the most effective anti-inflammatory properties.
Conclusions: SQE exhibited intestinal anti-inflammatory activity by inhibiting various inflammatory mediators mediated through nuclear transcription factor kappa-B (NF-kB) activation. Thus, SQE has the potential to ameliorate inflammation-related diseases, including IBD, by limiting excessive production of pro-inflammatory mediators.
Keywords: Sasa quelpaertensis; anti-inflammation; co-culture model; pro-inflammatory mediators.
Figures
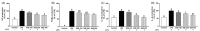
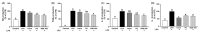

References
-
- Arai F, Takahashi T, Furukawa K, Matsushima K, Asakura H. Mucosal expression of interleukin-6 and interleukin-8 messenger RNA in ulcerative colitis and in Crohn's disease. Dig Dis Sci. 1998;43:2071–2079. - PubMed
-
- Loftus EV, Jr, Sandborn WJ. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:1–20. - PubMed
-
- Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. - PubMed
-
- Menditto A, Menotti A, Morisi G, Patriarca M, Spagnolo A. Serum ascorbic acid levels in men aged 55-75 years: association to selected social factors and biochemical parameters. Arch Gerontol Geriatr. 1992;15(Suppl 1):257–265. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

