Dual destructive and protective roles of adaptive immunity in neurodegenerative disorders
- PMID: 25671101
- PMCID: PMC4323229
- DOI: 10.1186/2047-9158-3-25
Dual destructive and protective roles of adaptive immunity in neurodegenerative disorders
Abstract
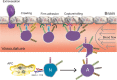
Inappropriate T cell responses in the central nervous system (CNS) affect the pathogenesis of a broad range of neuroinflammatory and neurodegenerative disorders that include, but are not limited to, multiple sclerosis, amyotrophic lateral sclerosis, Alzheimer's disease and Parkinson's disease. On the one hand immune responses can exacerbate neurotoxic responses; while on the other hand, they can lead to neuroprotective outcomes. The temporal and spatial mechanisms by which these immune responses occur and are regulated in the setting of active disease have gained significant recent attention. Spatially, immune responses that affect neurodegeneration may occur within or outside the CNS. Migration of antigen-specific CD4+ T cells from the periphery to the CNS and consequent immune cell interactions with resident glial cells affect neuroinflammation and neuronal survival. The destructive or protective mechanisms of these interactions are linked to the relative numerical and functional dominance of effector or regulatory T cells. Temporally, immune responses at disease onset or during progression may exhibit a differential balance of immune responses in the periphery and within the CNS. Immune responses with predominate T cell subtypes may differentially manifest migratory, regulatory and effector functions when triggered by endogenous misfolded and aggregated proteins and cell-specific stimuli. The final result is altered glial and neuronal behaviors that influence the disease course. Thus, discovery of neurodestructive and neuroprotective immune mechanisms will permit potential new therapeutic pathways that affect neuronal survival and slow disease progression.
Keywords: Effector T cell; MCAM; MPTP; Migration; Neurodegeneration; Neuroinflammation; Neuroprotection; Regulatory T cell.
Figures




References
-
- Pachter JS, de Vries HE, Fabry Z. The blood–brain barrier and its role in immune privilege in the central nervous system. J Neuropathol Exp Neurol. 2003;62:593–604. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

