Osteopontin: a leading candidate adhesion molecule for implantation in pigs and sheep
- PMID: 25671104
- PMCID: PMC4322467
- DOI: 10.1186/2049-1891-5-56
Osteopontin: a leading candidate adhesion molecule for implantation in pigs and sheep
Abstract
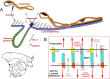
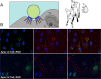
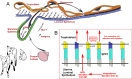
Osteopontin (OPN; also known as Secreted Phosphoprotein 1, SPP1) is a secreted extra-cellular matrix (ECM) protein that binds to a variety of cell surface integrins to stimulate cell-cell and cell-ECM adhesion and communication. It is generally accepted that OPN interacts with apically expressed integrin receptors on the uterine luminal epithelium (LE) and conceptus trophectoderm to attach the conceptus to the uterus for implantation. Research conducted with pigs and sheep has significantly advanced understanding of the role(s) of OPN during implantation through exploitation of the prolonged peri-implantation period of pregnancy when elongating conceptuses are free within the uterine lumen requiring extensive paracrine signaling between conceptus and endometrium. This is followed by a protracted and incremental attachment cascade of trophectoderm to uterine LE during implantation, and development of a true epitheliochorial or synepitheliochorial placenta exhibited by pigs and sheep, respectively. In pigs, implanting conceptuses secrete estrogens which induce the synthesis and secretion of OPN in adjacent uterine LE. OPN then binds to αvβ6 integrin receptors on trophectoderm, and the αvβ3 integrin receptors on uterine LE to bridge conceptus attachment to uterine LE for implantation. In sheep, implanting conceptuses secrete interferon tau that prolongs the lifespan of CL. Progesterone released by CL then induces OPN synthesis and secretion from the endometrial GE into the uterine lumen where OPN binds integrins expressed on trophectoderm (αvβ3) and uterine LE (identity of specific integrins unknown) to adhere the conceptus to the uterus for implantation. OPN binding to the αvβ3 integrin receptor on ovine trophectoderm cells induces in vitro focal adhesion assembly, a prerequisite for adhesion and migration of trophectoderm, through activation of: 1) P70S6K via crosstalk between FRAP1/MTOR and MAPK pathways; 2) MTOR, PI3K, MAPK3/MAPK1 (Erk1/2) and MAPK14 (p38) signaling to stimulate trohectoderm cell migration; and 3) focal adhesion assembly and myosin II motor activity to induce migration of trophectoderm cells. Further large in vivo focal adhesions assemble at the uterine-placental interface of both pigs and sheep and identify the involvement of sizable mechanical forces at this interface during discrete periods of trophoblast migration, attachment and placentation in both species.
Keywords: Implantation; Integrins; Pigs; Psteoponti; Sheep.
Figures

Similar articles
-
Secreted phosphoprotein 1 binds integrins to initiate multiple cell signaling pathways, including FRAP1/mTOR, to support attachment and force-generated migration of trophectoderm cells.Matrix Biol. 2010 Jun;29(5):369-82. doi: 10.1016/j.matbio.2010.04.001. Epub 2010 Apr 10. Matrix Biol. 2010. PMID: 20385232
-
Muc-1, integrin, and osteopontin expression during the implantation cascade in sheep.Biol Reprod. 2001 Sep;65(3):820-8. doi: 10.1095/biolreprod65.3.820. Biol Reprod. 2001. PMID: 11514347
-
Analysis of osteopontin at the maternal-placental interface in pigs.Biol Reprod. 2002 Mar;66(3):718-25. doi: 10.1095/biolreprod66.3.718. Biol Reprod. 2002. PMID: 11870079
-
Uterine histotroph and conceptus development: select nutrients and secreted phosphoprotein 1 affect mechanistic target of rapamycin cell signaling in ewes.Biol Reprod. 2011 Dec;85(6):1094-107. doi: 10.1095/biolreprod.111.094722. Epub 2011 Aug 24. Biol Reprod. 2011. PMID: 21865556 Review.
-
Biology of progesterone action during pregnancy recognition and maintenance of pregnancy.Front Biosci. 2002 Sep 1;7:d1879-98. doi: 10.2741/spencer. Front Biosci. 2002. PMID: 12161340 Review.
Cited by
-
MAML1: a coregulator that alters endometrial epithelial cell adhesive capacity.Fertil Res Pract. 2021 Mar 27;7(1):8. doi: 10.1186/s40738-021-00100-y. Fertil Res Pract. 2021. PMID: 33773601 Free PMC article.
-
Uterine Luminal Epithelium as the Transient Gateway for Embryo Implantation.Trends Endocrinol Metab. 2020 Feb;31(2):165-180. doi: 10.1016/j.tem.2019.11.008. Epub 2019 Dec 20. Trends Endocrinol Metab. 2020. PMID: 31866217 Free PMC article. Review.
-
Loss of PRICKLE1 leads to abnormal endometrial epithelial architecture, decreased embryo implantation, and reduced fertility in mice.PNAS Nexus. 2025 Jan 24;4(2):pgaf024. doi: 10.1093/pnasnexus/pgaf024. eCollection 2025 Feb. PNAS Nexus. 2025. PMID: 39917256 Free PMC article.
-
Identification and Analysis of Genes Related to Testicular Size in 14-Day-Old Piglets.Animals (Basel). 2024 Jan 4;14(1):172. doi: 10.3390/ani14010172. Animals (Basel). 2024. PMID: 38200903 Free PMC article.
-
Interleukin-15 deficient rats have reduced osteopontin at the maternal-fetal interface.Front Cell Dev Biol. 2023 Apr 20;11:1079164. doi: 10.3389/fcell.2023.1079164. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37152295 Free PMC article.
References
-
- Denhardt DT, Guo X. Osteopontin: a protein with diverse functions. FASEB J. 1993;7:1475–1482. - PubMed
-
- Butler WT, Ridall AL, McKee MD. Principles of Bone Biology. New York: Academic Press, Inc; 1996. Osteopontin; pp. 167–181.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

