Mitochondrial reactive oxygen species and cancer
- PMID: 25671107
- PMCID: PMC4323058
- DOI: 10.1186/2049-3002-2-17
Mitochondrial reactive oxygen species and cancer
Abstract
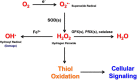
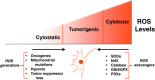
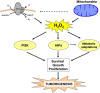
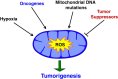
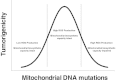
Mitochondria produce reactive oxygen species (mROS) as a natural by-product of electron transport chain activity. While initial studies focused on the damaging effects of reactive oxygen species, a recent paradigm shift has shown that mROS can act as signaling molecules to activate pro-growth responses. Cancer cells have long been observed to have increased production of ROS relative to normal cells, although the implications of this increase were not always clear. This is especially interesting considering cancer cells often also induce expression of antioxidant proteins. Here, we discuss how cancer-associated mutations and microenvironments can increase production of mROS, which can lead to activation of tumorigenic signaling and metabolic reprogramming. This tumorigenic signaling also increases expression of antioxidant proteins to balance the high production of ROS to maintain redox homeostasis. We also discuss how cancer-specific modifications to ROS and antioxidants may be targeted for therapy.
Keywords: Antioxidants; Cancer; Metabolism; Mitochondria reactive oxygen species; Oxidative stress; ROS.
Figures

References
-
- Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Can Res. 1991;51(3):794–798. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

