First noninvasive thermal ablation of a brain tumor with MR-guided focused ultrasound
- PMID: 25671132
- PMCID: PMC4322509
- DOI: 10.1186/2050-5736-2-17
First noninvasive thermal ablation of a brain tumor with MR-guided focused ultrasound
Abstract
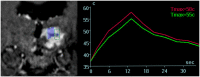
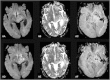
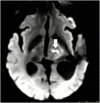
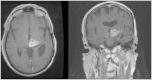
Magnetic resonance-guided focused ultrasound surgery (MRgFUS) allows for precise thermal ablation of target tissues. While this emerging modality is increasingly used for the treatment of various types of extracranial soft tissue tumors, it has only recently been acknowledged as a modality for noninvasive neurosurgery. MRgFUS has been particularly successful for functional neurosurgery, whereas its clinical application for tumor neurosurgery has been delayed for various technical and procedural reasons. Here, we report the case of a 63-year-old patient presenting with a centrally located recurrent glioblastoma who was included in our ongoing clinical phase I study aimed at evaluating the feasibility and safety of transcranial MRgFUS for brain tumor ablation. Applying 25 high-power sonications under MR imaging guidance, partial tumor ablation could be achieved without provoking neurological deficits or other adverse effects in the patient. This proves, for the first time, the feasibility of using transcranial MR-guided focused ultrasound to safely ablate substantial volumes of brain tumor tissue.
Keywords: Brain tumor; Focused ultrasound; HIFU; MRgFUS; Thermal ablation; Transcranial.
Figures

References
-
- Jagannathan J, Sanghvi NT, Crum LA, Yen CP, Medel R, Dumont AS, Sheehan JP, Steiner L, Jolesz F, Kassell NF. High-intensity focused ultrasound surgery of the brain: part 1–A historical perspective with modern applications. Neurosurgery. 2009;64(2):201–10. doi: 10.1227/01.NEU.0000336766.18197.8E. discussion 10–1. - DOI - PMC - PubMed
-
- Elias WJ, Huss D, Voss T, Loomba J, Khaled M, Zadicario E, Frysinger RC, Sperling SA, Wylie S, Monteith SJ, Druzgal J, Shah BB, Harrison M, Wintermark M. A pilot study of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2013;369(7):640–48. doi: 10.1056/NEJMoa1300962. - DOI - PubMed
-
- Jeanmonod D, Magara A, Kowalski M, Buler R, Payam P. Study of incisionless transcranial magnetic resonance-guided focused ultrasound treatment of Parkinson’s disesase: Safety, accuracy and initial outcomes 3rd International Symposium on Focused Ultrasound. Washington, DC;
LinkOut - more resources
Full Text Sources
Other Literature Sources

