Caveolin-1 and CDC42 mediated endocytosis of silica-coated iron oxide nanoparticles in HeLa cells
- PMID: 25671161
- PMCID: PMC4311761
- DOI: 10.3762/bjnano.6.16
Caveolin-1 and CDC42 mediated endocytosis of silica-coated iron oxide nanoparticles in HeLa cells
Abstract
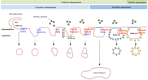
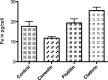
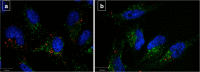
Nanomedicine is a rapidly growing field in nanotechnology, which has great potential in the development of new therapies for numerous diseases. For example iron oxide nanoparticles are in clinical use already in the thermotherapy of brain cancer. Although it has been shown, that tumor cells take up these particles in vitro, little is known about the internalization routes. Understanding of the underlying uptake mechanisms would be very useful for faster and precise development of nanoparticles for clinical applications. This study aims at the identification of key proteins, which are crucial for the active uptake of iron oxide nanoparticles by HeLa cells (human cervical cancer) as a model cell line. Cells were transfected with specific siRNAs against Caveolin-1, Dynamin 2, Flotillin-1, Clathrin, PIP5Kα and CDC42. Knockdown of Caveolin-1 reduces endocytosis of superparamagnetic iron oxide nanoparticles (SPIONs) and silica-coated iron oxide nanoparticles (SCIONs) between 23 and 41%, depending on the surface characteristics of the nanoparticles and the experimental design. Knockdown of CDC42 showed a 46% decrease of the internalization of PEGylated SPIONs within 24 h incubation time. Knockdown of Dynamin 2, Flotillin-1, Clathrin and PIP5Kα caused no or only minor effects. Hence endocytosis in HeLa cells of iron oxide nanoparticles, used in this study, is mainly mediated by Caveolin-1 and CDC42. It is shown here for the first time, which proteins of the endocytotic pathway mediate the endocytosis of silica-coated iron oxide nanoparticles in HeLa cells in vitro. In future studies more experiments should be carried out with different cell lines and other well-defined nanoparticle species to elucidate possible general principles.
Keywords: CDC42; Caveolin-1; endocytosis inhibition; iron oxide nanoparticles; nanoparticle uptake.
Figures






Similar articles
-
Increased endocytosis rate and enhanced lysosomal pathway of silica-coated superparamagnetic nanoparticles into M-HeLa cells compared with cultured primary motor neurons.Histochem Cell Biol. 2024 Jun;161(6):507-519. doi: 10.1007/s00418-024-02283-z. Epub 2024 Apr 10. Histochem Cell Biol. 2024. PMID: 38597938
-
Different endocytotic uptake mechanisms for nanoparticles in epithelial cells and macrophages.Beilstein J Nanotechnol. 2014 Sep 24;5:1625-36. doi: 10.3762/bjnano.5.174. eCollection 2014. Beilstein J Nanotechnol. 2014. PMID: 25383275 Free PMC article.
-
Clathrin and caveolin-1 expression in primary pigmented rabbit conjunctival epithelial cells: role in PLGA nanoparticle endocytosis.Mol Vis. 2003 Oct 15;9:559-68. Mol Vis. 2003. PMID: 14566223
-
Development of superparamagnetic iron oxide nanoparticles (SPIONS) for translation to clinical applications.IEEE Trans Nanobioscience. 2008 Dec;7(4):298-305. doi: 10.1109/TNB.2008.2011864. IEEE Trans Nanobioscience. 2008. PMID: 19203873 Review.
-
Superparamagnetic Iron Oxide Nanoparticles-Current and Prospective Medical Applications.Materials (Basel). 2019 Feb 19;12(4):617. doi: 10.3390/ma12040617. Materials (Basel). 2019. PMID: 30791358 Free PMC article. Review.
Cited by
-
Endocytosis: The Nanoparticle and Submicron Nanocompounds Gateway into the Cell.Pharmaceutics. 2020 Apr 17;12(4):371. doi: 10.3390/pharmaceutics12040371. Pharmaceutics. 2020. PMID: 32316537 Free PMC article. Review.
-
Intracellular Iron Binding and Antioxidant Activity of Phytochelators.Biol Trace Elem Res. 2022 Aug;200(8):3910-3918. doi: 10.1007/s12011-021-02965-y. Epub 2021 Oct 14. Biol Trace Elem Res. 2022. PMID: 34648123
-
Nanomaterials in Medicine: Understanding Cellular Uptake, Localization, and Retention for Enhanced Disease Diagnosis and Therapy.Aging Dis. 2024 Feb 22;16(1):168-208. doi: 10.14336/AD.2024.0206-1. Online ahead of print. Aging Dis. 2024. PMID: 38421835 Free PMC article. Review.
-
Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine.Chem Soc Rev. 2021 May 7;50(9):5397-5434. doi: 10.1039/d0cs01127d. Epub 2021 Mar 5. Chem Soc Rev. 2021. PMID: 33666625 Free PMC article. Review.
-
Enhanced Delivery and Potency of Chemotherapeutics in Melanoma Treatment via Magnetite Nanobioconjugates.ACS Omega. 2024 Oct 30;9(45):45402-45420. doi: 10.1021/acsomega.4c07415. eCollection 2024 Nov 12. ACS Omega. 2024. PMID: 39554413 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
