Human T cell crosstalk is induced by tumor membrane transfer
- PMID: 25671577
- PMCID: PMC4324967
- DOI: 10.1371/journal.pone.0118244
Human T cell crosstalk is induced by tumor membrane transfer
Abstract
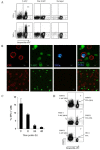
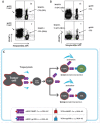
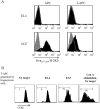
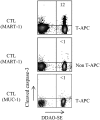
Trogocytosis is a contact-dependent unidirectional transfer of membrane fragments between immune effector cells and their targets, initially detected in T cells following interaction with professional antigen presenting cells (APC). Previously, we have demonstrated that trogocytosis also takes place between melanoma-specific cytotoxic T lymphocytes (CTLs) and their cognate tumors. In the present study, we took this finding a step further, focusing on the ability of melanoma membrane-imprinted CD8+ T cells to act as APCs (CD8+ T-APCs). We demonstrate that, following trogocytosis, CD8+ T-APCs directly present a variety of melanoma derived peptides to fraternal T cells with the same TCR specificity or to T cells with different TCRs. The resulting T cell-T cell immune synapse leads to (1) Activation of effector CTLs, as determined by proliferation, cytokine secretion and degranulation; (2) Fratricide (killing) of CD8+ T-APCs by the activated CTLs. Thus, trogocytosis enables cross-reactivity among CD8+ T cells with interchanging roles of effectors and APCs. This dual function of tumor-reactive CTLs may hint at their ability to amplify or restrict reactivity against the tumor and participate in modulation of the anti-cancer immune response.
Conflict of interest statement
Figures

References
-
- Joly E, Hudrisier D (2003) What is trogocytosis and what is its purpose? Nat Immunol 4: 815 - PubMed
-
- Hudrisier D, Riond J, Mazarguil H, Gairin JE, Joly E (2001) Cutting edge: CTLs rapidly capture membrane fragments from target cells in a TCR signaling-dependent manner. J Immunol 166: 3645–3649. - PubMed
-
- Espinosa E, Tabiasco J, Hudrisier D, Fournié JJ (2002) Synaptic transfer by human gamma delta T cells stimulated with soluble or cellular antigens. J Immunol 168: 6336–6343. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

