Immunological profiling of tuberculosis-associated immune reconstitution inflammatory syndrome and non-immune reconstitution inflammatory syndrome death in HIV-infected adults with pulmonary tuberculosis starting antiretroviral therapy: a prospective observational cohort study
- PMID: 25672566
- PMCID: PMC4624391
- DOI: 10.1016/S1473-3099(15)70008-3
Immunological profiling of tuberculosis-associated immune reconstitution inflammatory syndrome and non-immune reconstitution inflammatory syndrome death in HIV-infected adults with pulmonary tuberculosis starting antiretroviral therapy: a prospective observational cohort study
Erratum in
-
Corrections.Lancet Infect Dis. 2015 May;15(5):505. doi: 10.1016/S1473-3099(15)70170-2. Epub 2015 Apr 19. Lancet Infect Dis. 2015. PMID: 25932576
Abstract
Background: Patients co-infected with advanced HIV and tuberculosis are at risk of tuberculosis-associated immune reconstitution inflammatory syndrome (IRIS) and death soon after initiation of antiretroviral therapy (ART). Tuberculosis-associated IRIS has been associated with quicker recovery of cellular immune responses after ART initiation and early mortality with slower recovery of these responses. We aimed to assess whether patients who have these outcomes have distinct immunological profiles before and after ART initiation.
Methods: We undertook this prospective cohort study at 22 public clinics and the main public hospital in Gaborone, Botswana, in ART-naive adults (aged ≥21 years) with advanced HIV (CD4 cell counts ≤125 cells per μL) and pulmonary tuberculosis. We obtained data for clinical variables and for levels of 29 plasma biomarkers, quantified by Luminex assay. We classified patients as having tuberculosis-associated IRIS, early mortality, or survival without a diagnosis of tuberculosis-associated IRIS (controls), on the basis of outcomes recorded in the 6 months after ART initiation. We used rank-sum or χ(2) tests, and logistic regression with odds ratios (OR) and 95% CIs, to assess the association between variables measured before and 4 weeks after ART initiation with death and tuberculosis-associated IRIS, compared with controls.
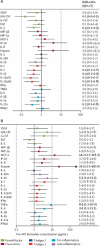
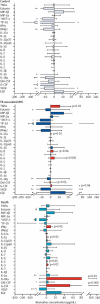
Findings: Between Nov 12, 2009, and July 3, 2013, we enrolled 201 participants. 31 (15%) patients left the study before ART initiation, leaving 170 (85%) patients for analysis. Patients with tuberculosis-associated IRIS had reduced pre-ART concentrations of several pro-inflammatory biomarkers, including interleukin (IL)-6 (adjusted OR per 1 log10 increase 0·40 [95% CI 0·18-0·89]). However, patients with early death had increased pre-ART concentrations of inflammatory biomarkers, including monocyte chemoattractant protein-1 (adjusted OR 9·0 [95% CI 1·0-80·0]) and tumour necrosis factor (TNF)α (7·8 [1·1-55·2]). At week 4 after ART initation, tuberculosis-associated IRIS was independently associated with greater increases in several inflammatory biomarkers, including IL-6 (adjusted OR 1·7 [95% CI 1·2-2·5]) and TNFα (1·5 [1·0-2·2]), versus controls. Death was likewise associated with greater increases in systemic inflammatory markers, including granulocyte colony-stimulating factor (adjusted OR 2·8 [95% CI 1·3-6·1]), IL-12p40 (1·8 [1·0-3·4]), and IL-15 (2·0 [1·1-3·7]), versus controls. However, changes in CD4 cell count during ART, which were similar between controls and patients with tuberculosis-associated IRIS (p=0·45), were substantially lower in patients who died (p=0·006).
Interpretation: Distinct immunological profiles before and after ART initiation characterise patients with advanced HIV and tuberculosis who have tuberculosis-associated IRIS and death. Interventions that decrease inflammation while promoting cellular immune recovery during ART should be considered in patients co-infected with HIV and tuberculosis.
Funding: National Institutes of Health and the Penn Center for AIDS Research.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Tuberculosis-associated immune reconstitution inflammatory syndrome: a manifestation of adaptive or innate immunity?Lancet Infect Dis. 2015 Apr;15(4):370-1. doi: 10.1016/S1473-3099(15)70026-5. Epub 2015 Feb 9. Lancet Infect Dis. 2015. PMID: 25672567 No abstract available.
References
-
- (WHO) Global Tuberculosis Report. WHO; 2014. 2014. [Dec 15, 2014]. Available: http://www.who.int/tb/publications/global_report/gtbr14_executive_summar....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

