The European medicines agency review of pomalidomide in combination with low-dose dexamethasone for the treatment of adult patients with multiple myeloma: summary of the scientific assessment of the committee for medicinal products for human use
- PMID: 25673103
- PMCID: PMC4350811
- DOI: 10.1634/theoncologist.2014-0073
The European medicines agency review of pomalidomide in combination with low-dose dexamethasone for the treatment of adult patients with multiple myeloma: summary of the scientific assessment of the committee for medicinal products for human use
Abstract
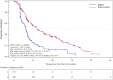
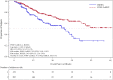
On August 5, 2013, a marketing authorization valid throughout the European Union (EU) was issued for pomalidomide in combination with dexamethasone for the treatment of adult patients with relapsed and refractory multiple myeloma (MM) who have received at least two prior treatment regimens, including both lenalidomide and bortezomib, and have demonstrated disease progression on the last therapy. Pomalidomide is an immunomodulating agent. The recommended starting dose of pomalidomide is 4 mg once daily taken on days 1-21 of repeated 28-day cycles. The main evidence of efficacy for pomalidomide in MM was based on a phase III multicenter, randomized, open-label study (CC-4047-MM-003) in which pomalidomide plus low-dose dexamethasone therapy (POM+LoDEX) was compared with high-dose dexamethasone alone (HiDEX) in previously treated adult patients with relapsed and refractory multiple myeloma who had received at least two prior treatment regimens, including both lenalidomide and bortezomib, and had demonstrated disease progression on the last therapy. For the intent-to-treat population, median progression-free survival based on International Myeloma Working Group criteria was 15.7 weeks (95% confidence interval [CI]: 13.0-20.1) in the POM+LoDEX group versus 8.0 weeks (95% CI: 7.0-9.0) in the HiDEX group (log-rank p value <.001). Overall survival (secondary endpoint) was also different in the two treatment groups (hazard ratio 0.53 [95% CI: 0.37-0.74]). The most commonly reported adverse reactions to pomalidomide in clinical studies were anemia (45.7%), neutropenia (45.3%) and thrombocytopenia (27%), fatigue (28.3%), pyrexia (21%), peripheral edema (13%), and infections including pneumonia (10.7%). Peripheral neuropathy adverse reactions were reported in 12.3% of patients, and venous embolic or thrombotic (VTE) adverse reactions were reported in 3.3% of patients. Pomalidomide is expected to be teratogenic. This paper summarizes the scientific review of the application leading to approval in the EU. The detailed scientific assessment report and product information, including the summary of product characteristics, are available on the EMA website (http://www.ema.europa.eu).
Keywords: EMA; European Medicines Agency; Imnovid; Multiple myeloma; Pomalidomide.
©AlphaMed Press.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
References
-
- Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860–1873. - PubMed
-
- Hideshima T, Chauhan D, Shima Y, et al. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood. 2000;96:2943–2950. - PubMed
-
- Corral LG, Haslett PA, Muller GW, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol. 1999;163:380–386. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

