New genetic loci link adipose and insulin biology to body fat distribution
- PMID: 25673412
- PMCID: PMC4338562
- DOI: 10.1038/nature14132
New genetic loci link adipose and insulin biology to body fat distribution
Abstract
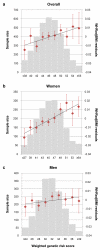
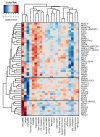
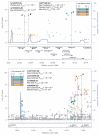
Body fat distribution is a heritable trait and a well-established predictor of adverse metabolic outcomes, independent of overall adiposity. To increase our understanding of the genetic basis of body fat distribution and its molecular links to cardiometabolic traits, here we conduct genome-wide association meta-analyses of traits related to waist and hip circumferences in up to 224,459 individuals. We identify 49 loci (33 new) associated with waist-to-hip ratio adjusted for body mass index (BMI), and an additional 19 loci newly associated with related waist and hip circumference measures (P < 5 × 10(-8)). In total, 20 of the 49 waist-to-hip ratio adjusted for BMI loci show significant sexual dimorphism, 19 of which display a stronger effect in women. The identified loci were enriched for genes expressed in adipose tissue and for putative regulatory elements in adipocytes. Pathway analyses implicated adipogenesis, angiogenesis, transcriptional regulation and insulin resistance as processes affecting fat distribution, providing insight into potential pathophysiological mechanisms.
Figures


Comment in
-
Apple or pear: size and shape matter.Cell Metab. 2015 Apr 7;21(4):507-8. doi: 10.1016/j.cmet.2015.03.016. Cell Metab. 2015. PMID: 25863240
References
-
- Pischon T, et al. General and abdominal adiposity and risk of death in Europe. N. Engl. J. Med. 2008;359:2105–2120. - PubMed
-
- Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am. J. Clin. Nutr. 2005;81:555–563. - PubMed
-
- Canoy D. Distribution of body fat and risk of coronary heart disease in men and women. Curr. Opin. Cardiol. 2008;23:591–598. - PubMed
-
- Snijder MB, et al. Associations of hip and thigh circumferences independent of waist circumference with the incidence of type 2 diabetes: the Hoorn Study. Am. J. Clin. Nutr. 2003;77:1192–1197. - PubMed
-
- Yusuf S, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–1649. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL120393/HL/NHLBI NIH HHS/United States
- 085235/WT_/Wellcome Trust/United Kingdom
- R01 DK075787/DK/NIDDK NIH HHS/United States
- MRC_MC_UP_A620_1014/MRC_/Medical Research Council/United Kingdom
- R01 DK093757/DK/NIDDK NIH HHS/United States
- U01 HG007417/HG/NHGRI NIH HHS/United States
- MC_UU_12012/1/MRC_/Medical Research Council/United Kingdom
- BHF_RG/13/2/30098/BHF_/British Heart Foundation/United Kingdom
- CZB/4/710/CSO_/Chief Scientist Office/United Kingdom
- G9521010/MRC_/Medical Research Council/United Kingdom
- G1001799/MRC_/Medical Research Council/United Kingdom
- G0601463/MRC_/Medical Research Council/United Kingdom
- R21 DA027040/DA/NIDA NIH HHS/United States
- K23 DK080145/DK/NIDDK NIH HHS/United States
- 098017/WT_/Wellcome Trust/United Kingdom
- RG/13/2/30098/BHF_/British Heart Foundation/United Kingdom
- P30 DK040561/DK/NIDDK NIH HHS/United States
- U01 HG007419/HG/NHGRI NIH HHS/United States
- RG/08/008/25291/BHF_/British Heart Foundation/United Kingdom
- WT097117/WT_/Wellcome Trust/United Kingdom
- MC_U147585827/MRC_/Medical Research Council/United Kingdom
- BHF_RG/08/014/24067/BHF_/British Heart Foundation/United Kingdom
- Z01 HG000024/ImNIH/Intramural NIH HHS/United States
- R01 HL109946/HL/NHLBI NIH HHS/United States
- MC_UP_A100_1003/MRC_/Medical Research Council/United Kingdom
- MRC_MC_UU_12012/1/MRC_/Medical Research Council/United Kingdom
- WT098498/WT_/Wellcome Trust/United Kingdom
- 12/0004470/DUK_/Diabetes UK/United Kingdom
- G19/35/MRC_/Medical Research Council/United Kingdom
- T32 GM067553/GM/NIGMS NIH HHS/United States
- G0100222/MRC_/Medical Research Council/United Kingdom
- MRC_MC_UU_12015/1/MRC_/Medical Research Council/United Kingdom
- U01 HG007416/HG/NHGRI NIH HHS/United States
- MRC_G1001799/MRC_/Medical Research Council/United Kingdom
- WT084766/WT_/Wellcome Trust/United Kingdom
- RG/08/014/24067/BHF_/British Heart Foundation/United Kingdom
- MRC_G9521010/MRC_/Medical Research Council/United Kingdom
- R01 DK072193/DK/NIDDK NIH HHS/United States
- P30 DK079637/DK/NIDDK NIH HHS/United States
- MRC_G0401527/MRC_/Medical Research Council/United Kingdom
- MRC_MC_UU_12015/5/MRC_/Medical Research Council/United Kingdom
- MC_UU_12019/1/MRC_/Medical Research Council/United Kingdom
- G8802774/MRC_/Medical Research Council/United Kingdom
- P30 DK056341/DK/NIDDK NIH HHS/United States
- MC_U147585819/MRC_/Medical Research Council/United Kingdom
- G1000143/MRC_/Medical Research Council/United Kingdom
- U01 HG007376/HG/NHGRI NIH HHS/United States
- WT098381/WT_/Wellcome Trust/United Kingdom
- G0902037/MRC_/Medical Research Council/United Kingdom
- MR/L003120/1/MRC_/Medical Research Council/United Kingdom
- R01 AG025941/AG/NIA NIH HHS/United States
- R01 AG041517/AG/NIA NIH HHS/United States
- MRC_MC_PC_U127561128/MRC_/Medical Research Council/United Kingdom
- U54 HD083211/HD/NICHD NIH HHS/United States
- MC_UU_12015/1/MRC_/Medical Research Council/United Kingdom
- R01 DK107437/DK/NIDDK NIH HHS/United States
- MRC_MC_UU_12015/2/MRC_/Medical Research Council/United Kingdom
- MC_UP_A620_1014/MRC_/Medical Research Council/United Kingdom
- R01 DK062370/DK/NIDDK NIH HHS/United States
- R01 HL105756/HL/NHLBI NIH HHS/United States
- MC_U106179471/MRC_/Medical Research Council/United Kingdom
- MR/N01104X/1/MRC_/Medical Research Council/United Kingdom
- CSO_CZB/4/710/CSO_/Chief Scientist Office/United Kingdom
- 097117/WT_/Wellcome Trust/United Kingdom
- WT098017/WT_/Wellcome Trust/United Kingdom
- MC_UU_12015/2/MRC_/Medical Research Council/United Kingdom
- 098498/WT_/Wellcome Trust/United Kingdom
- P20 MD006899/MD/NIMHD NIH HHS/United States
- P30 DK072488/DK/NIDDK NIH HHS/United States
- K01 HL116770/HL/NHLBI NIH HHS/United States
- 14136/CRUK_/Cancer Research UK/United Kingdom
- R01 AG033193/AG/NIA NIH HHS/United States
- G1000616/MRC_/Medical Research Council/United Kingdom
- MRC_MC_UP_A100_1003/MRC_/Medical Research Council/United Kingdom
- MC_U106179472/MRC_/Medical Research Council/United Kingdom
- G0401527/MRC_/Medical Research Council/United Kingdom
- MRC_G0601261/MRC_/Medical Research Council/United Kingdom
- MC_UU_12013/1/MRC_/Medical Research Council/United Kingdom
- BHF_RG/08/008/25291/BHF_/British Heart Foundation/United Kingdom
- R00 HL094535/HL/NHLBI NIH HHS/United States
- P30 DK020541/DK/NIDDK NIH HHS/United States
- MC_UU_12013/4/MRC_/Medical Research Council/United Kingdom
- MC_PC_U127561128/MRC_/Medical Research Council/United Kingdom
- UM1 CA182913/CA/NCI NIH HHS/United States
- U01 DK062370/DK/NIDDK NIH HHS/United States
- G0600717/MRC_/Medical Research Council/United Kingdom
- WT100574/WT_/Wellcome Trust/United Kingdom
- T32 GM007092/GM/NIGMS NIH HHS/United States
- MRC_MC_UU_12013/1/MRC_/Medical Research Council/United Kingdom
- 084766/WT_/Wellcome Trust/United Kingdom
- MRC_MR/N01104X/1/MRC_/Medical Research Council/United Kingdom
- 100574/WT_/Wellcome Trust/United Kingdom
- MRC_G0601463/MRC_/Medical Research Council/United Kingdom
- MRC_MC_U106179472/MRC_/Medical Research Council/United Kingdom
- FS/14/55/30806/BHF_/British Heart Foundation/United Kingdom
- P30 GM103341/GM/NIGMS NIH HHS/United States
- R01 DK089256/DK/NIDDK NIH HHS/United States
- MR/K006584/1/MRC_/Medical Research Council/United Kingdom
- MR/K013351/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_12011/1/MRC_/Medical Research Council/United Kingdom
- BHF_FS/14/55/30806/BHF_/British Heart Foundation/United Kingdom
- MRC_MC_U106179471/MRC_/Medical Research Council/United Kingdom
- CRUK_14136/CRUK_/Cancer Research UK/United Kingdom
- T32 HL069768/HL/NHLBI NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- MC_UU_12015/5/MRC_/Medical Research Council/United Kingdom
- MRC_MR/K013351/1/MRC_/Medical Research Council/United Kingdom
- P60 DK020541/DK/NIDDK NIH HHS/United States
- R01 HL117626/HL/NHLBI NIH HHS/United States
- 098381/WT_/Wellcome Trust/United Kingdom
- R01 HL117078/HL/NHLBI NIH HHS/United States
- WT085235/WT_/Wellcome Trust/United Kingdom
- MRC_G1000143/MRC_/Medical Research Council/United Kingdom
- MR/K011480/1/MRC_/Medical Research Council/United Kingdom
- G0400491/MRC_/Medical Research Council/United Kingdom
- UM1 CA182910/CA/NCI NIH HHS/United States
- T32 HL007055/HL/NHLBI NIH HHS/United States
- MRC_MR/L003120/1/MRC_/Medical Research Council/United Kingdom
- G0601261/MRC_/Medical Research Council/United Kingdom
- P30 CA071789/CA/NCI NIH HHS/United States
- MRC_MC_UU_12011/1/MRC_/Medical Research Council/United Kingdom
- MC_U147585824/MRC_/Medical Research Council/United Kingdom
- MRC_MC_UU_12019/1/MRC_/Medical Research Council/United Kingdom
- BHF_RG/10/12/28456/BHF_/British Heart Foundation/United Kingdom
- R01 DK078150/DK/NIDDK NIH HHS/United States
- DUK_12/0004470/DUK_/Diabetes UK/United Kingdom
- MRC_MR/K006584/1/MRC_/Medical Research Council/United Kingdom
- MRC_MR/K011480/1/MRC_/Medical Research Council/United Kingdom
- U01 AG049505/AG/NIA NIH HHS/United States
- RG/07/008/23674/BHF_/British Heart Foundation/United Kingdom
- RG/10/12/28456/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

