Efficacy and safety of Tripterygium wilfordii hook F versus acitretin in moderate to severe psoriasis vulgaris: a randomized clinical trial
- PMID: 25673443
- PMCID: PMC4836244
- DOI: 10.4103/0366-6999.151069
Efficacy and safety of Tripterygium wilfordii hook F versus acitretin in moderate to severe psoriasis vulgaris: a randomized clinical trial
Abstract
Background: Few clinical trials have evaluated the efficacy and safety of Tripterygium wilfordii Hook F (TwHF) compared with acitretin in psoriasis. We aimed to compare the efficacy and safety of TwHF compared with acitretin in the treatment of moderate to severe psoriasis vulgaris.
Methods: Adults with Psoriasis Area Severity Index (PASI) score ≥ 10 and psoriasis-affected body surface area ≥ 10% were randomized into a TwHF (20 mg, 3 times a day) or acitretin group (30 mg, once a day). The treatment course lasted for 8 weeks. Patients were assessed at baseline and at 2, 4, and 8 weeks. Laboratory tests were performed at baseline, week 4, and week 8. The data were analyzed using paired samples t-test or analysis of variance (ANOVA).
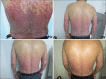
Results: A total of 115 patients was enrolled (58 TwHF; 57 acitretin). The median PASI score improved in the TwHF group by 50.4% and in the acitretin group by 42.7%. There was no significant difference in median PASI improvement between two groups at 2, 4, and 8 weeks. There was also no significant difference in PASI 25, PASI 50, PASI 75, and PASI 90 response between the two groups at 2, 4, and 8 weeks. There was a significant increase in the level of aspartate transaminase and triglycerides in the TwHF group (P = 0.026 and P = 0.011, respectively). In the acitretin group, there was a significant increase in the level of alanine transaminase, cholesterol, and high-density lipoprotein (P = 0.030, P < 0.01, and P < 0.01, respectively).
Conclusions: There was no significant difference in treatment efficacy between the TwHF and acitretin groups within 8 weeks, but there were fewer treatment-related adverse events in the TwHF group.
Conflict of interest statement
Figures


References
-
- Swimberghe S, Ghislain PD, Daci E, Allewaert K, Denhaerynck K, Hermans C, et al. Clinical, quality of life, patient adherence, and safety outcomes of short-course (12 weeks) treatment with cyclosporine in patients with severe psoriasis (the practice study) Ann Dermatol. 2013;25:28–35. - PMC - PubMed
-
- Ren J, Wu X, Liao N, Wang G, Fan C, Liu S, et al. Prevention of postoperative recurrence of Crohn's disease: Tripterygium wilfordii polyglycoside versus mesalazine. J Int Med Res. 2013;41:176–87. - PubMed
-
- Tao XL, Sun Y, Dong Y, Xiao YL, Hu DW, Shi YP, et al. A prospective, controlled, double-blind, cross-over study of Tripterygium wilfodii Hook F in treatment of rheumatoid arthritis. Chin Med J. 1989;102:327–32. - PubMed
-
- Gu WZ, Brandwein SR. Inhibition of type II collagen-induced arthritis in rats by triptolide. Int J Immunopharmacol. 1998;20:389–400. - PubMed
-
- Qin WZ, Liu CH, Yang SM, Zhu GD, Han KY, Fang L, et al. Tripterygium wilfordii Hook F in systemic lupus erythematosus: Report of 103 cases. Chin Med J. 1981;94:827–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

