The effects on bronchial epithelial mucociliary cultures of coarse, fine, and ultrafine particulate matter from an underground railway station
- PMID: 25673499
- PMCID: PMC4408962
- DOI: 10.1093/toxsci/kfv034
The effects on bronchial epithelial mucociliary cultures of coarse, fine, and ultrafine particulate matter from an underground railway station
Abstract
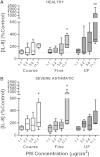
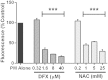
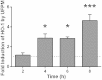
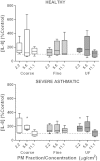
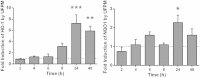
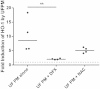
We have previously shown that underground railway particulate matter (PM) is rich in iron and other transition metals across coarse (PM10-2.5), fine (PM2.5), and quasi-ultrafine (PM0.18) fractions and is able to generate reactive oxygen species (ROS). However, there is little knowledge of whether the metal-rich nature of such particles exerts toxic effects in mucus-covered airway epithelial cell cultures or whether there is an increased risk posed by the ultrafine fraction. Monolayer and mucociliary air-liquid interface (ALI) cultures of primary bronchial epithelial cells (PBECs) were exposed to size-fractionated underground railway PM (1.1-11.1 µg/cm(2)) and release of lactate dehydrogenase and IL-8 was assayed. ROS generation was measured, and the mechanism of generation studied using desferrioxamine (DFX) and N-acetylcysteine (NAC). Expression of heme oxygenase-1 (HO-1) was determined by RT-qPCR. Particle uptake was studied by transmission electron microscopy. Underground PM increased IL-8 release from PBECs, but this was diminished in mucus-secreting ALI cultures. Fine and ultrafine PM generated a greater level of ROS than coarse PM. ROS generation by ultrafine PM was ameliorated by DFX and NAC, suggesting an iron-dependent mechanism. Despite the presence of mucus, ALI cultures displayed increased HO-1 expression. Intracellular PM was observed within vesicles, mitochondria, and free in the cytosol. The results indicate that, although the mucous layer appears to confer some protection against underground PM, ALI PBECs nonetheless detect PM and mount an antioxidant response. The combination of increased ROS-generating ability of the metal-rich ultrafine fraction and ability of PM to penetrate the mucous layer merits further research.
Keywords: bronchial epithelium; environmental exposure; metals; particulate matter; primary cell culture; underground railway.
© The Author 2015. Published by Oxford University Press on behalf of the Society of Toxicology.
Figures


References
-
- Bachoual R., Boczkowski J., Goven D., Amara N., Tabet L., On D., Lecon-Malas V., Aubier M., Lanone S. (2007). Biological effects of particles from the Paris subway system. Chem. Res. Toxicol. 20, 1426–1433. - PubMed
-
- Baulig A., Garlatti M., Bonvallot V., Marchand A., Barouki R., Marano F., Baeza-Squiban A. (2003). Involvement of reactive oxygen species in the metabolic pathways triggered by diesel exhaust particles in human airway epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 285, L671–L679. - PubMed
-
- Belade E., Armand L., Martinon L., Kheuang L., Fleury-Feith J., Baeza-Squiban A., Lanone S., Billon-Galland M. A., Pairon J. C., Boczkowski J. (2012). A comparative transmission electron microscopy study of titanium dioxide and carbon black nanoparticles uptake in human lung epithelial and fibroblast cell lines. Toxicol. in vitro 26, 57-66. - PubMed
-
- Bigert C., Alderling M., Svartengren M., Plato N., de Faire U., Gustavsson P. (2008). Blood markers of inflammation and coagulation and exposure to airborne particles in employees in the Stockholm underground. Occup. Environ. Med. 65, 655-658. - PubMed
-
- Bigert C., Alderling M., Svartengren M., Plato N., Gustavsson P. (2011). No short-term respiratory effects among particle-exposed employees in the Stockholm subway. Scand. J. Work. Environ. Health 37, 129-135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

