High-fat diet causes bone loss in young mice by promoting osteoclastogenesis through alteration of the bone marrow environment
- PMID: 25673503
- PMCID: PMC4383048
- DOI: 10.1007/s00223-015-9954-z
High-fat diet causes bone loss in young mice by promoting osteoclastogenesis through alteration of the bone marrow environment
Abstract
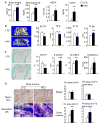
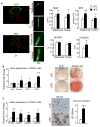
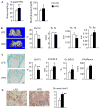
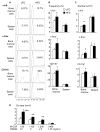
Obesity is a severe health problem in children, afflicting several organ systems including bone. However, the role of obesity on bone homeostasis and bone cell function in children has not been studied in detail. Here we used young mice fed a high-fat diet (HFD) to model childhood obesity and investigate the effect of HFD on the phenotype of cells within the bone marrow environment. Five-week-old male mice were fed a HFD for 3, 6, and 12 weeks. Decreased bone volume was detected after 3 weeks of HFD treatment. After 6 and 12 weeks, HFD-exposed mice had less bone mass and increased osteoclast numbers. Bone marrow cells, but not spleen cells, from HFD-fed mice had increased osteoclast precursor frequency, elevated osteoclast formation, and bone resorption activity, as well as increased expression of osteoclastogenic regulators including RANKL, TNF, and PPAR-gamma. Bone formation rate and osteoblast and adipocyte numbers were also increased in HFD-fed mice. Isolated bone marrow cells also had a corresponding elevation in the expression of positive regulators of osteoblast and adipocyte differentiation. Our findings indicate that in juvenile mice, HFD-induced bone loss is mainly due to increased osteoclast bone resorption by affecting the bone marrow microenvironment. Thus, targeting osteoclast formation may present a new therapeutic approach for bone complications in obese children.
Figures


References
-
- Dimitri P, Bishop N, Walsh JS, Eastell R. Obesity is a risk factor for fracture in children but is protective against fracture in adults: a paradox. Bone. 2012;50:457–466. - PubMed
-
- Looker AC, Flegal KM, Melton LJ., 3rd Impact of increased overweight on the projected prevalence of osteoporosis in older women. Osteoporos Int. 2007;18:307–313. - PubMed
-
- Albala C, Yanez M, Devoto E, Sostin C, Zeballos L, Santos JL. Obesity as a protective factor for postmenopausal osteoporosis. Int J Obes Relat Metab Disord. 1996;20:1027–1032. - PubMed
-
- Cao JJ, Gregoire BR, Gao H. High-fat diet decreases cancellous bone mass but has no effect on cortical bone mass in the tibia in mice. Bone. 2009;44:1097–1104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

