Evaluation of a minimal sedation protocol using ICU sedative consumption as a monitoring tool: a quality improvement multicenter project
- PMID: 25673553
- PMCID: PMC4234844
- DOI: 10.1186/s13054-014-0580-3
Evaluation of a minimal sedation protocol using ICU sedative consumption as a monitoring tool: a quality improvement multicenter project
Abstract
Introduction: Oversedation frequently occurs in ICUs. We aimed to evaluate a minimal sedation policy, using sedative consumption as a monitoring tool, in a network of ICUs targeting decrement of oversedation and mechanical ventilation (MV) duration.
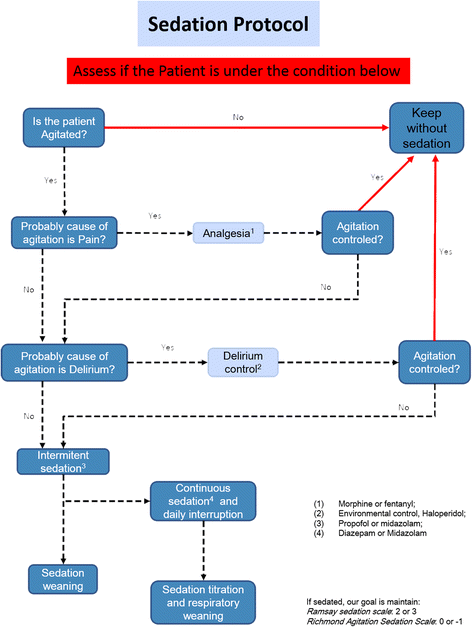
Methods: A prospective quality improvement project was conducted in ten ICUs within a network of nonteaching hospitals in Brazil during a 2-year period (2010 to 2012). In the first 12 months (the preintervention period), we conducted an audit to identify sedation practice and barriers to current guideline-based practice regarding sedation. In the postintervention period, we implemented a multifaceted program, including multidisciplinary daily rounds, and monthly audits focusing on sedative consumption, feedback and benchmarking purposes. To analyze the effect of the campaign, we fit an interrupted time series (ITS). To account for variability among the network ICUs, we fit a hierarchical model.
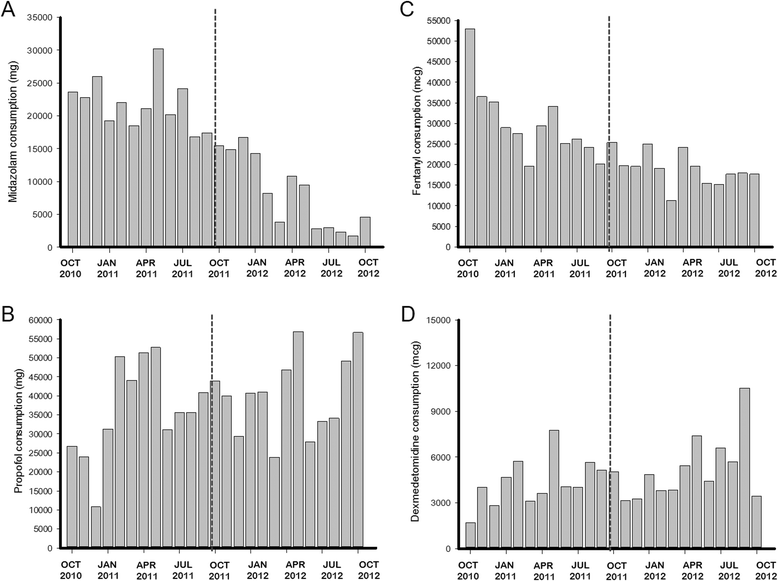
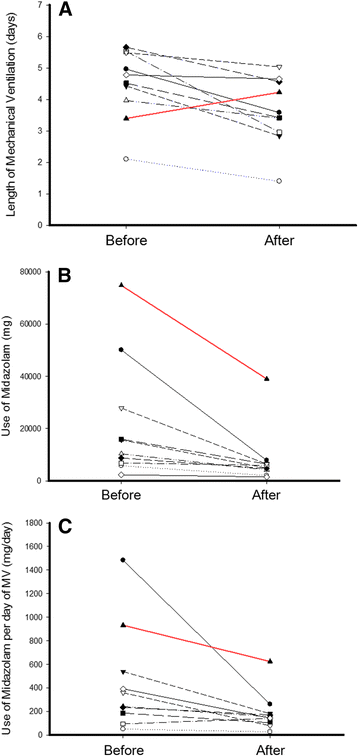
Results: During the study period, 21% of patients received MV (4,851/22,963). In the postintervention period, the length of MV was lower (3.91 ± 6.2 days versus 3.15 ± 4.6 days; mean difference, -0.76 (95% CI, -1.10; -0.43), P <0.001) and 28 ventilator-free days were higher (16.07 ± 12.2 days versus 18.33 ± 11.6 days; mean difference, 2.30 (95% CI, 1.57; 3.00), P <0.001) than in the preintervention period. Midazolam consumption (in milligrams per day of MV) decreased from 329 ± 70 mg/day to 163 ± 115 mg/day (mean difference, -167 (95% CI, -246; -87), P <0.001). In contrast, consumption of propofol (P = 0.007), dexmedetomidine (P = 0.017) and haloperidol (P = 0.002) increased in the postintervention period, without changes in the consumption of fentanyl. Through ITS, age (P = 0.574) and Simplified Acute Physiology Score III (P = 0.176) remained stable. The length of MV showed a secular effect (secular trend β(1) = -0.055, P = 0.012) and a strong decrease immediately after the intervention (intervention β(2) = -0.976, P <0.001). The impact was maintained over the course of one year, despite the waning trend for the intervention's effect (postintervention trend β(3)= 0.039, P = 0.095).
Conclusions: By using a light sedation policy in a group of nonteaching hospitals, we reproduced the benefits that have previously been demonstrated in controlled settings. Furthermore, systematic monitoring of sedative consumption should be a feasible instrument for supporting the implementation of a protocol on a large scale.
Figures



References
-
- Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM, Coursin DB, Herr DL, Tung A, Robinson BR, Fontaine DK, Ramsay MA, Riker RR, Sessler CN, Pun B, Skrobik Y, Jaeschke R. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. - DOI - PubMed
-
- Salluh JI, Soares M, Teles JM, Ceraso D, Raimondi N, Nava VS, Blasquez P, Ugarte S, Ibanez-Guzman C, Centeno JV, Laca M, Grecco G, Jimenez E, Árias-Rivera S, Duenas C, Rocha MG, the DECCA (Delirium Epidemiology in Critical Care) Study Group Delirium epidemiology in critical care (DECCA): an international study. Crit Care. 2010;14:R210. doi: 10.1186/cc9333. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

