A Translational, Pharmacodynamic, and Pharmacokinetic Phase IB Clinical Study of Everolimus in Resectable Non-Small Cell Lung Cancer
- PMID: 25673697
- PMCID: PMC4401630
- DOI: 10.1158/1078-0432.CCR-14-1998
A Translational, Pharmacodynamic, and Pharmacokinetic Phase IB Clinical Study of Everolimus in Resectable Non-Small Cell Lung Cancer
Abstract
Purpose: The altered PI3K/mTOR pathway is implicated in lung cancer, but mTOR inhibitors have failed to demonstrate efficacy in advanced lung cancer. We studied the pharmacodynamic effects of everolimus in resectable non-small cell lung cancer (NSCLC) to inform further development of these agents in lung cancer.
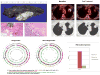
Experimental design: We enrolled 33 patients and obtained baseline tumor biopsy and 2[18F]fluoro-2-deoxy-D-glucose-positron emission tomography/computed tomography (FDG-PET/CT) imaging followed by everolimus treatment (5 or 10 mg daily, up to 28 days), or without intervening treatment for controls. Target modulation by everolimus was quantified in vivo and ex vivo by comparing metabolic activity on paired PET scans and expression of active phosphorylated forms of mTOR, Akt, S6, eIF4e, p70S6K, 4EBP1, and total Bim protein between pretreatment and posttreatment tissue samples.
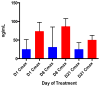
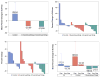
Results: There were 23 patients on the treatment arm and 10 controls; median age 64 years; 22 tumors (67%) were adenocarcinomas. There was a dose-dependent reduction in metabolic activity (SUVmax: 29.0%, -21%, -24%; P = 0.014), tumor size (10.1%, 5.8%, -11.6%; P = 0.047), and modulation of S6 (-36.1, -13.7, -77.0; P = 0.071) and pS6 (-41.25, -61.57, -47.21; P = 0.063) in patients treated in the control, 5-mg, and 10-mg cohorts, respectively. Targeted DNA sequencing in all patients along with exome and whole transcriptome RNA-seq in an index patient with hypersensitive tumor was employed to further elucidate the mechanism of everolimus activity.
Conclusions: This "window-of-opportunity" study demonstrated measurable, dose-dependent, biologic, metabolic, and antitumor activity of everolimus in early-stage NSCLC.
©2015 American Association for Cancer Research.
Conflict of interest statement
No significant conflicts reported by the authors.
Figures
References
-
- Tsao AS, McDonnell T, Lam S, Putnam JB, Bekele N, Hong WK, et al. Increased phospho-AKT (Ser(473)) expression in bronchial dysplasia: implications for lung cancer prevention studies. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive. Oncology. 2003;12:660–4. - PubMed
-
- Balsara BR, Pei J, Mitsuuchi Y, Page R, Klein-Szanto A, Wang H, et al. Frequent activation of AKT in non-small cell lung carcinomas and preneoplastic bronchial lesions. Carcinogenesis. 2004;25:2053–9. - PubMed
-
- Sanchez-Cespedes M, Parrella P, Esteller M, Nomoto S, Trink B, Engles JM, et al. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 2002;62:3659–62. - PubMed
-
- Ramalingam SS, Owonikoko TK, Behera M, Subramanian J, Saba NF, Kono SA, et al. Phase II study of docetaxel in combination with everolimus for second- or third-line therapy of advanced non-small-cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2013;8:369–72. - PMC - PubMed
-
- Milton DT, Riely GJ, Azzoli CG, Gomez JE, Heelan RT, Kris MG, et al. Phase 1 trial of everolimus and gefitinib in patients with advanced nonsmall-cell lung cancer. Cancer. 2007;110:599–605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

