The acetyltransferase Tip60 is a critical regulator of the differentiation-dependent amplification of human papillomaviruses
- PMID: 25673709
- PMCID: PMC4442364
- DOI: 10.1128/JVI.03455-14
The acetyltransferase Tip60 is a critical regulator of the differentiation-dependent amplification of human papillomaviruses
Abstract
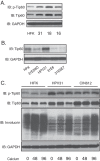
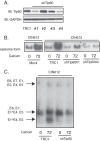
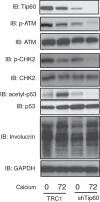
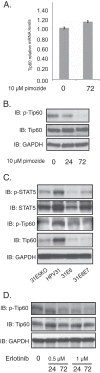
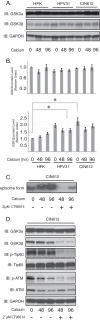
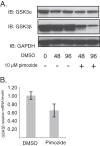
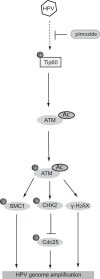
The life cycle of human papillomaviruses (HPVs) is dependent upon differentiation of the infected host epithelial cell as well as activation of the ataxia telangiectasia mutated (ATM) DNA repair pathway that in normal cells acts to repair double-strand DNA breaks. In normal cells, following DNA damage the acetyltransferase Tip60 must acetylate ATM proteins prior to their full activation by autophosphorylation. E6 proteins have been shown to induce the degradation of Tip60, suggesting that Tip60 action may not be required for activation of the ATM pathway in HPV-positive cells. We investigated what role, if any, Tip60 plays in regulating the differentiation-dependent HPV life cycle. Our study indicates that Tip60 levels and activity are increased in cells that stably maintain complete HPV genomes as episomes, while low levels are seen in cells that express only HPV E6 and E7 proteins. Knockdown of Tip60 with short hairpin RNAs in cells that maintain HPV episomes blocked ATM induction and differentiation-dependent genome amplification, demonstrating the critical role of Tip60 in the viral life cycle. The JAK/STAT transcription factor STAT-5 has previously been shown to regulate the phosphorylation of ATM. Our studies demonstrate that STAT-5 regulates Tip60 activation and this occurs in part by targeting glycogen synthase kinase 3β (GSK3β). Inhibition of either STAT-5, Tip60, or GSK3β blocked differentiation-dependent genome amplification. Taken together, our findings identify Tip60 to be an important regulator of HPV genome amplification whose activity during the viral life cycle is controlled by STAT-5 and the kinase GSK3β.
Importance: Human papillomaviruses (HPVs) are the etiological agents of cervical and other anogenital cancers. HPVs regulate their differentiation-dependent life cycle by activation of DNA damage pathways. This study demonstrates that HPVs regulate the ATM DNA damage pathway through the action of the acetyltransferase Tip60. Furthermore, the innate immune regulator STAT-5 and the kinase GSK3β mediate the activation of Tip60 in HPV-positive cells. This study identifies critical regulators of the HPV life cycle.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
Regulation of the Human Papillomavirus Life Cycle by DNA Damage Repair Pathways and Epigenetic Factors.Viruses. 2020 Jul 10;12(7):744. doi: 10.3390/v12070744. Viruses. 2020. PMID: 32664381 Free PMC article. Review.
-
STAT-5 Regulates Transcription of the Topoisomerase IIβ-Binding Protein 1 (TopBP1) Gene To Activate the ATR Pathway and Promote Human Papillomavirus Replication.mBio. 2015 Dec 22;6(6):e02006-15. doi: 10.1128/mBio.02006-15. mBio. 2015. PMID: 26695634 Free PMC article.
-
The JAK-STAT transcriptional regulator, STAT-5, activates the ATM DNA damage pathway to induce HPV 31 genome amplification upon epithelial differentiation.PLoS Pathog. 2013;9(4):e1003295. doi: 10.1371/journal.ppat.1003295. Epub 2013 Apr 4. PLoS Pathog. 2013. PMID: 23593005 Free PMC article.
-
Human papillomaviruses activate the ATM DNA damage pathway for viral genome amplification upon differentiation.PLoS Pathog. 2009 Oct;5(10):e1000605. doi: 10.1371/journal.ppat.1000605. Epub 2009 Oct 2. PLoS Pathog. 2009. PMID: 19798429 Free PMC article.
-
DNA damage response is hijacked by human papillomaviruses to complete their life cycle.J Zhejiang Univ Sci B. 2017 Mar;18(3):215-232. doi: 10.1631/jzus.B1600306. J Zhejiang Univ Sci B. 2017. PMID: 28271657 Free PMC article. Review.
Cited by
-
Persistent Human Papillomavirus Infection.Viruses. 2021 Feb 20;13(2):321. doi: 10.3390/v13020321. Viruses. 2021. PMID: 33672465 Free PMC article. Review.
-
Antagonistic Relationship between Human Cytomegalovirus pUL27 and pUL97 Activities during Infection.J Virol. 2015 Oct;89(20):10230-46. doi: 10.1128/JVI.00986-15. Epub 2015 Jul 29. J Virol. 2015. PMID: 26223645 Free PMC article.
-
Regulation of the Human Papillomavirus Life Cycle by DNA Damage Repair Pathways and Epigenetic Factors.Viruses. 2020 Jul 10;12(7):744. doi: 10.3390/v12070744. Viruses. 2020. PMID: 32664381 Free PMC article. Review.
-
The SETD2 Methyltransferase Supports Productive HPV31 Replication through the LEDGF/CtIP/Rad51 Pathway.J Virol. 2023 May 31;97(5):e0020123. doi: 10.1128/jvi.00201-23. Epub 2023 May 8. J Virol. 2023. PMID: 37154769 Free PMC article.
-
Human papillomavirus type 18 E5 oncogene supports cell cycle progression and impairs epithelial differentiation by modulating growth factor receptor signalling during the virus life cycle.Oncotarget. 2017 Oct 6;8(61):103581-103600. doi: 10.18632/oncotarget.21658. eCollection 2017 Nov 28. Oncotarget. 2017. PMID: 29262586 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

