Triatoma virus recombinant VP4 protein induces membrane permeability through dynamic pores
- PMID: 25673713
- PMCID: PMC4442390
- DOI: 10.1128/JVI.00011-15
Triatoma virus recombinant VP4 protein induces membrane permeability through dynamic pores
Abstract
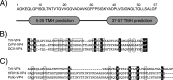
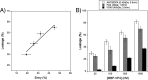
In naked viruses, membrane breaching is a key step that must be performed for genome transfer into the target cells. Despite its importance, the mechanisms behind this process remain poorly understood. The small protein VP4, encoded by the genomes of most viruses of the order Picornavirales, has been shown to be involved in membrane alterations. Here we analyzed the permeabilization activity of the natively nonmyristoylated VP4 protein from triatoma virus (TrV), a virus belonging to the Dicistroviridae family within the Picornavirales order. The VP4 protein was produced as a C-terminal maltose binding protein (MBP) fusion to achieve its successful expression. This recombinant VP4 protein is able to produce membrane permeabilization in model membranes in a membrane composition-dependent manner. The induced permeability was also influenced by the pH, being greater at higher pH values. We demonstrate that the permeabilization activity elicited by the protein occurs through discrete pores that are inserted on the membrane. Sizing experiments using fluorescent dextrans, cryo-electron microscopy imaging, and other, additional techniques showed that recombinant VP4 forms heterogeneous proteolipidic pores rather than common proteinaceous channels. These results suggest that the VP4 protein may be involved in the membrane alterations required for genome transfer or cell entry steps during dicistrovirus infection.
Importance: During viral infection, viruses need to overcome the membrane barrier in order to enter the cell and replicate their genome. In nonenveloped viruses membrane fusion is not possible, and hence, other mechanisms are implemented. Among other proteins, like the capsid-forming proteins and the proteins required for viral replication, several viruses of the order Picornaviridae contain a small protein called VP4 that has been shown to be involved in membrane alterations. Here we show that the triatoma virus VP4 protein is able to produce membrane permeabilization in model membranes by the formation of heterogeneous dynamic pores. These pores formed by VP4 may be involved in the genome transfer or cell entry steps during viral infection.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures





Similar articles
-
Cryo-electron Microscopy Study of the Genome Release of the Dicistrovirus Israeli Acute Bee Paralysis Virus.J Virol. 2017 Jan 31;91(4):e02060-16. doi: 10.1128/JVI.02060-16. Print 2017 Feb 15. J Virol. 2017. PMID: 27928006 Free PMC article.
-
Real-Time Imaging of Polioviral RNA Translocation across a Membrane.mBio. 2021 Feb 23;12(1):e03695-20. doi: 10.1128/mBio.03695-20. mBio. 2021. PMID: 33622727 Free PMC article.
-
Capsid protein VP4 of human rhinovirus induces membrane permeability by the formation of a size-selective multimeric pore.PLoS Pathog. 2014 Aug 7;10(8):e1004294. doi: 10.1371/journal.ppat.1004294. eCollection 2014 Aug. PLoS Pathog. 2014. PMID: 25102288 Free PMC article.
-
Breach: Host Membrane Penetration and Entry by Nonenveloped Viruses.Trends Microbiol. 2018 Jun;26(6):525-537. doi: 10.1016/j.tim.2017.09.010. Epub 2017 Oct 25. Trends Microbiol. 2018. PMID: 29079499 Review.
-
Rethinking the capsid proteins of enveloped viruses: multifunctionality from genome packaging to genome transfection.FEBS J. 2015 Jun;282(12):2267-78. doi: 10.1111/febs.13274. Epub 2015 Apr 11. FEBS J. 2015. PMID: 25808179 Review.
Cited by
-
Development of Monoclonal Antibody to Specifically Recognize VP0 but Not VP4 and VP2 of Foot-and-Mouth Disease Virus.Pathogens. 2022 Dec 8;11(12):1493. doi: 10.3390/pathogens11121493. Pathogens. 2022. PMID: 36558827 Free PMC article.
-
ICTV Virus Taxonomy Profile: Dicistroviridae.J Gen Virol. 2017 Mar;98(3):355-356. doi: 10.1099/jgv.0.000756. J Gen Virol. 2017. PMID: 28366189 Free PMC article.
-
Virion structure and in vitro genome release mechanism of dicistrovirus Kashmir bee virus.J Virol. 2021 May 10;95(11):e01950-20. doi: 10.1128/JVI.01950-20. Epub 2021 Mar 3. J Virol. 2021. PMID: 33658338 Free PMC article.
-
Targeting the Channel Activity of Viroporins.Adv Protein Chem Struct Biol. 2016;104:307-355. doi: 10.1016/bs.apcsb.2015.12.003. Epub 2016 Jan 7. Adv Protein Chem Struct Biol. 2016. PMID: 27038378 Free PMC article. Review.
-
Multiscale modelization in a small virus: Mechanism of proton channeling and its role in triggering capsid disassembly.PLoS Comput Biol. 2018 Apr 16;14(4):e1006082. doi: 10.1371/journal.pcbi.1006082. eCollection 2018 Apr. PLoS Comput Biol. 2018. PMID: 29659564 Free PMC article.
References
-
- Marti GA, Echeverría MG, Susevich ML, Ceccarelli S, Balsalobre A, Canale D, Stariolo R, Noireau F, García AL, González-Cifuentes NL, Guhl F, Bacigalupo A, Cattan PE, García A, Villacis AG, Grijalva MJ, Solorzano E, Monroy C, Espinoza-Blanco Y, Cordoca-Benzaquen E, Ruelas-Llerena N, Guzmán-Loayza M, Caceres AG, Vences-Blanco MO, Salazar-Schettino PM, Mojoli A, Rojas de Arias A, Felicaingeli MD, Rivera Mendoza P, Rozas-Dennis GS, Sánchez-Eugenia R, Agirre J, Viguera AR, Hernández-Suárez CM, Vilchez S, Osuna A, Gorla DE, Mougabure-Cueto G, Esteban L, Angulo VM, Querido JFB, Silva MS, Marques T, Gómez-Hernández C, Ramírez LE, Diotaiuti L, Rabinovich JE, Guérin DMA. 2013. Exploration for triatoma virus (TrV) infection in laboratory-reared triatomines of Latin America: a collaborative study. Int J Trop Insect Sci 33:294–304. doi:10.1017/S1742758413000337. - DOI
-
- Czibener C, La Torre JL, Muscio OA, Ugalde RA, Scodeller EA. 2000. Nucleotide sequence analysis of triatoma virus shows that it is a member of a novel group of insect RNA viruses. J Gen Virol 81:1149–1154. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

