An anti-H5N1 influenza virus FcDART antibody is a highly efficacious therapeutic agent and prophylactic against H5N1 influenza virus infection
- PMID: 25673719
- PMCID: PMC4442345
- DOI: 10.1128/JVI.00078-15
An anti-H5N1 influenza virus FcDART antibody is a highly efficacious therapeutic agent and prophylactic against H5N1 influenza virus infection
Abstract
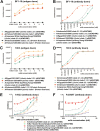
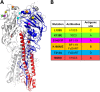
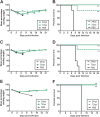
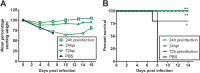
Highly pathogenic H5N1 avian influenza viruses are associated with severe disease in humans and continue to be a pandemic threat. While vaccines are available, other approaches are required for patients that typically respond poorly to vaccination, such as the elderly and the immunocompromised. To produce a therapeutic agent that is highly efficacious at low doses and is broadly specific against antigenically drifted H5N1 influenza viruses, we developed two neutralizing monoclonal antibodies and combined them into a single bispecific Fc fusion protein (the Fc dual-affinity retargeting [FcDART] molecule). In mice, a single therapeutic or prophylactic dose of either monoclonal antibody at 2.5 mg/kg of body weight provided 100% protection against challenge with A/Vietnam/1203/04 (H5N1) or the antigenically drifted strain A/Whooper swan/Mongolia/244/05 (H5N1). In ferrets, a single 1-mg/kg prophylactic dose provided 100% protection against A/Vietnam/1203/04 challenge. FcDART was also effective, as a single 2.5-mg/kg therapeutic or prophylactic dose in mice provided 100% protection against A/Vietnam/1203/04 challenge. Antibodies bound to conformational epitopes in antigenic sites on the globular head of the hemagglutinin protein, on the basis of analysis of mutants with antibody escape mutations. While it was possible to generate escape mutants in vitro, they were neutralized by the antibodies in vivo, as mice infected with escape mutants were 100% protected after only a single therapeutic dose of the antibody used to generate the escape mutant in vitro. In summary, we have combined the antigen specificities of two highly efficacious anti-H5N1 influenza virus antibodies into a bispecific FcDART molecule, which represents a strategy to produce broadly neutralizing antibodies that are effective against antigenically diverse influenza viruses.
Importance: Highly pathogenic H5N1 avian influenza viruses are associated with severe disease in humans and are a pandemic threat. A vaccine is available, but other approaches are required for patients that typically respond poorly to vaccination, such as the elderly and the immunocompromised. The variability of the virus means that such an approach must be broad spectrum. To achieve this, we developed two antibodies that neutralize H5N1 influenza viruses. In mice, these antibodies provided complete protection against a spectrum of H5N1 influenza viruses at a single low dose. We then combined the two antibodies into a single molecule, FcDART, which combined the broad-spectrum activity and protective efficacy of both antibodies. This treatment provides a novel and effective therapeutic agent or prophylactic with activity against highly pathogenic H5N1 avian influenza viruses.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures





References
-
- Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, Munster VJ, Sorrell EM, Bestebroer TM, Burke DF, Smith DJ, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. 2012. Airborne transmission of influenza A/H5N1 virus between ferrets. Science 336:1534–1541. doi:10.1126/science.1213362. - DOI - PMC - PubMed
-
- Imai M, Watanabe T, Hatta M, Das SC, Ozawa M, Shinya K, Zhong G, Hanson A, Katsura H, Watanabe S, Li C, Kawakami E, Yamada S, Kiso M, Suzuki Y, Maher EA, Neumann G, Kawaoka Y. 2012. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 486:420–428. doi:10.1038/nature10831. - DOI - PMC - PubMed
-
- Govorkova EA, Rehg JE, Krauss S, Yen HL, Guan Y, Peiris M, Nguyen TD, Hanh TH, Puthavathana P, Long HT, Buranathai C, Lim W, Webster RG, Hoffmann E. 2005. Lethality to ferrets of H5N1 influenza viruses isolated from humans and poultry in 2004. J Virol 79:2191–2198. doi:10.1128/JVI.79.4.2191-2198.2005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

