Glutamine-based PET imaging facilitates enhanced metabolic evaluation of gliomas in vivo
- PMID: 25673762
- PMCID: PMC4431550
- DOI: 10.1126/scitranslmed.aaa1009
Glutamine-based PET imaging facilitates enhanced metabolic evaluation of gliomas in vivo
Abstract
Glucose and glutamine are the two principal nutrients that cancer cells use to proliferate and survive. Many cancers show altered glucose metabolism, which constitutes the basis for in vivo positron emission tomography (PET) imaging with (18)F-fluorodeoxyglucose ((18)F-FDG). However, (18)F-FDG is ineffective in evaluating gliomas because of high background uptake in the brain. Glutamine metabolism is also altered in many cancers, and we demonstrate that PET imaging in vivo with the glutamine analog 4-(18)F-(2S,4R)-fluoroglutamine ((18)F-FGln) shows high uptake in gliomas but low background brain uptake, facilitating clear tumor delineation. Chemo/radiation therapy reduced (18)F-FGln tumor avidity, corresponding with decreased tumor burden. (18)F-FGln uptake was not observed in animals with a permeable blood-brain barrier or neuroinflammation. We translated these findings to human subjects, where (18)F-FGln showed high tumor/background ratios with minimal uptake in the surrounding brain in human glioma patients with progressive disease. These data suggest that (18)F-FGln is avidly taken up by gliomas, can be used to assess metabolic nutrient uptake in gliomas in vivo, and may serve as a valuable tool in the clinical management of gliomas.
Copyright © 2015, American Association for the Advancement of Science.
Conflict of interest statement
C.B.T is co-founder of Agios Pharmaceuticals and has financial interest in Agios. The other authors of this study declare that they have no competing interests.
Figures

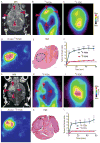
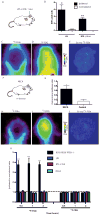

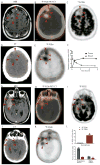
References
-
- Weber WA, Schwaiger M, Avril N. Quantitative assessment of tumor metabolism using FDG-PET imaging. Nuclear medicine and biology. 2000;27:683–687. - PubMed
-
- Klasner BD, Krause BJ, Beer AJ, Drzezga A. PET imaging of gliomas using novel tracers: a sleeping beauty waiting to be kissed. Expert review of anticancer therapy. 2010;10:609–613. - PubMed
-
- Petrirena GJ, Goldman S, Delattre JY. Advances in PET imaging of brain tumors: a referring physician’s perspective. Curr Opin Oncol. 2011;23:617–623. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

