Ubiquitin binding by the CUE domain promotes endosomal localization of the Rab5 GEF Vps9
- PMID: 25673804
- PMCID: PMC4454180
- DOI: 10.1091/mbc.E14-06-1156
Ubiquitin binding by the CUE domain promotes endosomal localization of the Rab5 GEF Vps9
Abstract
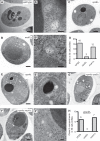
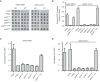
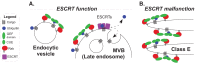
Vps9 and Muk1 are guanine nucleotide exchange factors (GEFs) in Saccharomyces cerevisiae that regulate membrane trafficking in the endolysosomal pathway by activating Rab5 GTPases. We show that Vps9 is the primary Rab5 GEF required for biogenesis of late endosomal multivesicular bodies (MVBs). However, only Vps9 (but not Muk1) is required for the formation of aberrant class E compartments that arise upon dysfunction of endosomal sorting complexes required for transport (ESCRTs). ESCRT dysfunction causes ubiquitinated transmembrane proteins to accumulate at endosomes, and we demonstrate that endosomal recruitment of Vps9 is promoted by its ubiquitin-binding CUE domain. Muk1 lacks ubiquitin-binding motifs, but its fusion to the Vps9 CUE domain allows Muk1 to rescue endosome morphology, cargo trafficking, and cellular stress-tolerance phenotypes that result from loss of Vps9 function. These results indicate that ubiquitin binding by the CUE domain promotes Vps9 function in endolysosomal membrane trafficking via promotion of localization.
© 2015 Shideler et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures

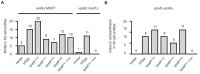

References
-
- Burd CG, Emr SD. Phosphatidylinositol (3)-phosphate signaling mediated by specific binding to RING FYVE domains. Mol Cell. 1998;2:157–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

