Control of axon guidance and neurotransmitter phenotype of dB1 hindbrain interneurons by Lim-HD code
- PMID: 25673852
- PMCID: PMC6605615
- DOI: 10.1523/JNEUROSCI.2699-14.2015
Control of axon guidance and neurotransmitter phenotype of dB1 hindbrain interneurons by Lim-HD code
Abstract
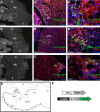
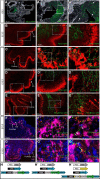
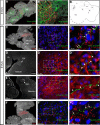
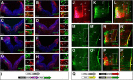
Hindbrain dorsal interneurons (HDIs) are implicated in receiving, processing, integrating, and transmitting sensory inputs from the periphery and spinal cord, including the vestibular, auditory, and proprioceptive systems. During development, multiple molecularly defined HDI types are set in columns along the dorsoventral axis, before migrating along well-defined trajectories to generate various brainstem nuclei. Major brainstem functions rely on the precise assembly of different interneuron groups and higher brain domains into common circuitries. Yet, knowledge regarding interneuron axonal patterns, synaptic targets, and the transcriptional control that govern their connectivity is sparse. The dB1 class of HDIs is formed in a district dorsomedial position along the hindbrain and gives rise to the inferior olive nuclei, dorsal cochlear nuclei, and vestibular nuclei. dB1 interneurons express various transcription factors (TFs): the pancreatic transcription factor 1a (Ptf1a), the homeobox TF-Lbx1 and the Lim-homeodomain (Lim-HD), and TF Lhx1 and Lhx5. To decipher the axonal and synaptic connectivity of dB1 cells, we have used advanced enhancer tools combined with conditional expression systems and the PiggyBac-mediated DNA transposition system in avian embryos. Multiple ipsilateral and contralateral axonal projections were identified ascending toward higher brain centers, where they formed synapses in the Purkinje cerebellar layer as well as at discrete midbrain auditory and vestibular centers. Decoding the mechanisms that instruct dB1 circuit formation revealed a fundamental role for Lim-HD proteins in regulating their axonal patterns, synaptic targets, and neurotransmitter choice. Together, this study provides new insights into the assembly and heterogeneity of HDIs connectivity and its establishment through the central action of Lim-HD governed programs.
Keywords: Lim-HD proteins; axons; hindbrain; interneurons; neurotransmitter; synapses.
Copyright © 2015 the authors 0270-6474/15/352596-16$15.00/0.
Figures



Similar articles
-
Axonal patterns and targets of dA1 interneurons in the chick hindbrain.J Neurosci. 2012 Apr 25;32(17):5757-71. doi: 10.1523/JNEUROSCI.4231-11.2012. J Neurosci. 2012. PMID: 22539838 Free PMC article.
-
Transcriptional control of axonal guidance and sorting in dorsal interneurons by the Lim-HD proteins Lhx9 and Lhx1.Neural Dev. 2009 Jun 19;4:21. doi: 10.1186/1749-8104-4-21. Neural Dev. 2009. PMID: 19545367 Free PMC article.
-
Electroporation of the hindbrain to trace axonal trajectories and synaptic targets in the chick embryo.J Vis Exp. 2013 May 29;(75):e50136. doi: 10.3791/50136. J Vis Exp. 2013. PMID: 23748440 Free PMC article.
-
Axonal Projection Patterns of the Dorsal Interneuron Populations in the Embryonic Hindbrain.Front Neuroanat. 2021 Dec 24;15:793161. doi: 10.3389/fnana.2021.793161. eCollection 2021. Front Neuroanat. 2021. PMID: 35002640 Free PMC article. Review.
-
Mechanisms of axonal guidance used by interneurons in the chick embryo spinal cord.Perspect Dev Neurobiol. 1993;1(4):205-15. Perspect Dev Neurobiol. 1993. PMID: 8087545 Review.
Cited by
-
Netrin1-DCC-Mediated Attraction Guides Post-Crossing Commissural Axons in the Hindbrain.J Neurosci. 2015 Aug 19;35(33):11707-18. doi: 10.1523/JNEUROSCI.0613-15.2015. J Neurosci. 2015. PMID: 26290247 Free PMC article.
-
Molecular Profiling Defines Evolutionarily Conserved Transcription Factor Signatures of Major Vestibulospinal Neuron Groups.eNeuro. 2019 Feb 27;6(1):ENEURO.0475-18.2019. doi: 10.1523/ENEURO.0475-18.2019. eCollection 2019 Jan-Feb. eNeuro. 2019. PMID: 30899776 Free PMC article.
-
Regeneration of Spinal Cord Connectivity Through Stem Cell Transplantation and Biomaterial Scaffolds.Front Cell Neurosci. 2019 Jun 6;13:248. doi: 10.3389/fncel.2019.00248. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31244609 Free PMC article. Review.
-
Temporal-specific roles of fragile X mental retardation protein in the development of the hindbrain auditory circuit.Development. 2020 Aug 25;147(21):dev188797. doi: 10.1242/dev.188797. Development. 2020. PMID: 32747436 Free PMC article.
-
Neural stem cells deriving from chick embryonic hindbrain recapitulate hindbrain development in culture.Sci Rep. 2018 Sep 17;8(1):13920. doi: 10.1038/s41598-018-32203-w. Sci Rep. 2018. PMID: 30224755 Free PMC article.
References
-
- Altman J, Bayer SA. Time of origin and distribution of a new cell type in the rat cerebellar cortex. Exp Brain Res. 1977;29:265–274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
